PVX_123682 heterochromatin protein 1, putative (HP1)
Disruptability [+]
| Species | Disruptability | Reference | Submitter | |
|---|---|---|---|---|
| P. falciparum 3D7 |
Refractory |
19270070 | Theo Sanderson, Wellcome Trust Sanger Institute | |
| P. falciparum 3D7 |
Refractory |
USF piggyBac screen (Insert. mut.) | USF PiggyBac Screen | |
Mutant phenotypes [+]
| Species | Stage | Phenotype | Reference | Submitter |
|---|---|---|---|---|
| P. berghei ANKA | Asexual |
Attenuated |
PlasmoGEM (Barseq) | PlasmoGEM |
| P. falciparum 3D7 | Asexual |
Transcriptional effect |
25121746 (Conditional)
\". Conditional depletion of P. falciparum HP1 (PfHP1) prevents mitotic proliferation of blood stage parasites and disrupts mutually exclusive expression and antigenic variation of the major virulence factor PfEMP1. Additionally, PfHP1-dependent regulation of PfAP2-G, a transcription factor required for gametocyte conversion, controls the switch from asexual proliferation to sexual differentiation, providing insight into the epigenetic mechanisms underlying gametocyte commitment. \" |
Theo Sanderson, Francis Crick Institute |
| P. falciparum 3D7 | Gametocyte |
Transcriptional effect |
25121746 (Conditional)
\". Conditional depletion of P. falciparum HP1 (PfHP1) prevents mitotic proliferation of blood stage parasites and disrupts mutually exclusive expression and antigenic variation of the major virulence factor PfEMP1. Additionally, PfHP1-dependent regulation of PfAP2-G, a transcription factor required for gametocyte conversion, controls the switch from asexual proliferation to sexual differentiation, providing insight into the epigenetic mechanisms underlying gametocyte commitment. \" |
Theo Sanderson, Francis Crick Institute |
Imaging data (from Malaria Metabolic Pathways)
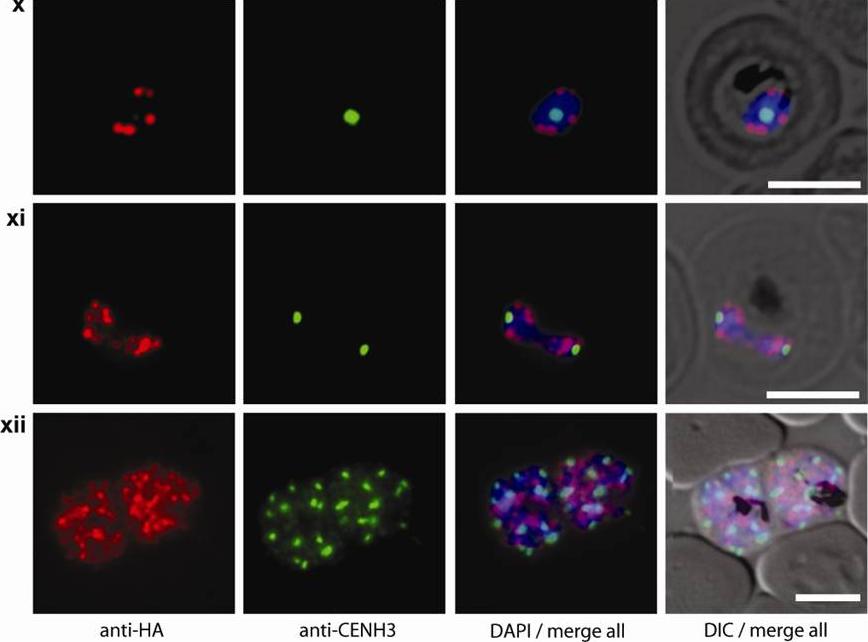
Double staining immunofluorescence pictures obtained from the transgenic parasite line 3D7/HP1-HA expressing HA-tagged PfHP1. Cells were simultaneously probed with aHA and aPfCENH3 antibodies. PfCENH3 foci do not overlap with PfHP1-marked chromosome end clusters. GFP live fluorescence and aPfCENH3 6 antibody (green), aHA antibody (red), DAPI nuclear stain (blue), DIC (differential interference contrast). Scale bars are 5 μm long.Hoeijmakers WA, Flueck C, Françoijs KJ, Smits AH, Wetzel J, Volz JC, Cowman AF, Voss T, Stunnenberg HG, Bártfai R. Plasmodium falciparum centromeres display a unique epigenetic makeup and cluster prior to and during schizogony. Cell Microbiol. 2012 14(9):1391-401.
See original on MMP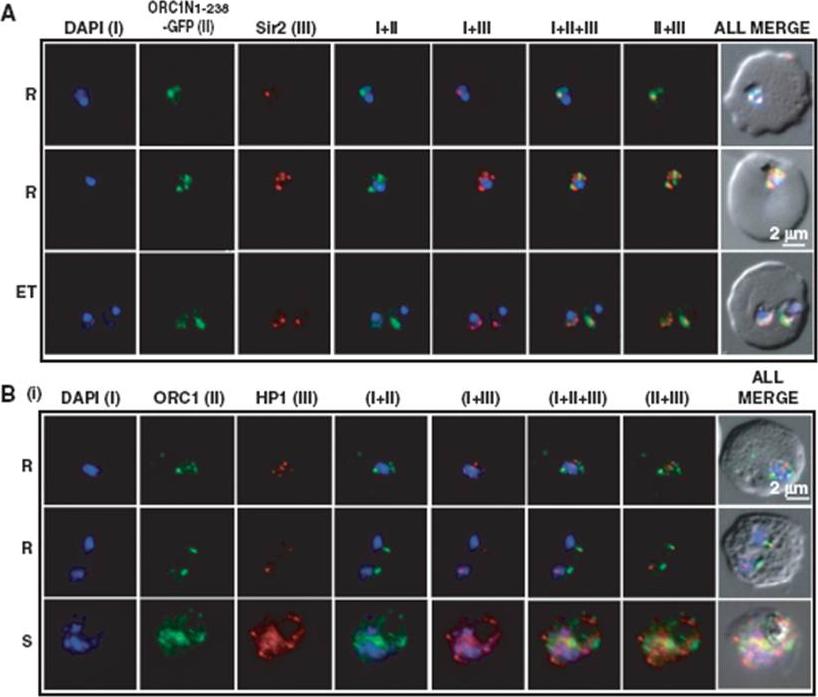
Co-localization of ORC1N1–238–GFP and Sir2 and immunolocalization of ORC1 and HP1 in 3D7. Glass slides containing the parasite smears from ORC1N1–238–GFP expressing parasites or 3D7 wild-type parasite lines were treated for immunolocalization using respective antibodies as indicated in the panels (A) and (B). (A) Co-localization of GFP and Sir2 in ORC1N1–238–GFP parasites during early stages of development as indicated on the left. ‘R’ indicates ring stage and ‘ET’ indicates early trophozoite stage parasites. DAPI shows the nuclei. Both ORC1 and Sir2 are localized to the nuclear periphery during the ring or early trophozoite stage. However, both proteins reorganize themselves at the onset of DNA replication and spread in the nucleus and cytoplasm. B(i): Both ORC1 and HP1 show nuclear punctate staining in 3D7 wild-type parasites during the early stages of development. ‘S’ indicates schizont stage parasites.Deshmukh AS, Srivastava S, Herrmann S, Gupta A, Mitra P, Gilberger TW, Dhar SK. The role of N-terminus of Plasmodium falciparum ORC1 in telomeric localization and var gene silencing. Nucleic Acids Res. 2012 40(12):5313-31
See original on MMP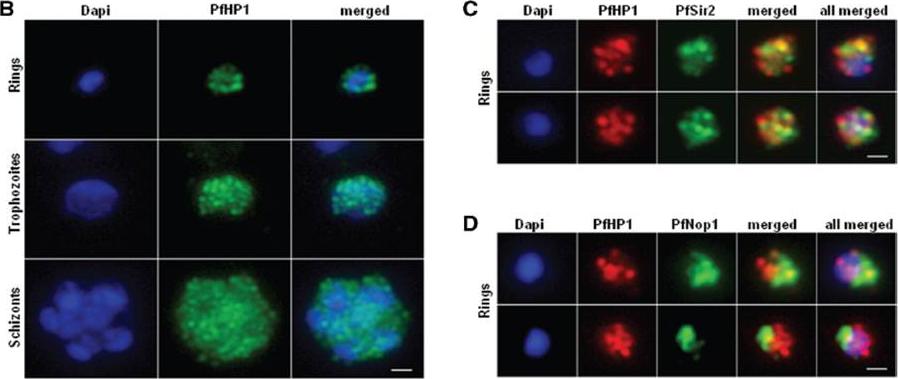
Nuclear expression and cellular localization of PfHP1. (B) IFA analysis of PfHP1 (green) throughout the parasite asexual blood stage cycle (ring, trophozoite and schizont stages). (C) In ring stage, partial co-localization between PfHP1 (red) and PfSir2 (green) antibodies (yellow spots) is observed. (D) Co-localization with a nucleolar marker PfNop1 shows minimal overlap of PfHP1 with this compartment. Scale bars represent 1 mm.Pérez-Toledo K, Rojas-Meza AP, Mancio-Silva L, Hernández-Cuevas NA, Delgadillo DM, Vargas M, Martínez-Calvillo S, Scherf A, Hernandez-Rivas R. Plasmodium falciparum heterochromatin protein 1 binds to tri-methylated histone 3 lysine 9 and is linked to mutually exclusive expression of var genes. Nucleic Acids Res. 2009 37(8):2596-606
See original on MMP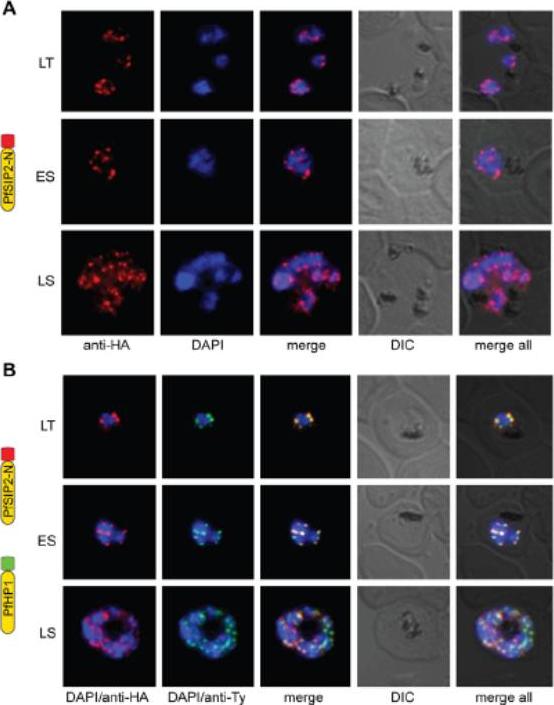
PfSIP2-N localizes to P. falciparum chromosome end clusters. (A) IFA detects discrete perinuclear PfSIP2-N-HA signals in late trophozoites (LT) and early (ES) and late (LS) schizont stage 3D7/SIP2-N-HA parasites. The expression cassette is schematically depicted on the left. (B) Co-localisation of PfSIP2-N with PfHP1-containing subtelomeric heterochromatin in late trophozoites (LT) and early (ES) and late (LS) schizonts in the double transgenic parasite line 3D7/SIP2-N-HA/HP1-Ty (over-expressing both proteins as epitope-tagged versions simultaneously). Expression cassettes are schematically depicted on the left. Indirect immunofluorescence (IFA) microscopy identified discrete PfSIP2-N-HA foci at the nuclear periphery with increasing numbers of foci in replicating stages (A) consistent with chromosome end clusters. PfHP1 is a heterochromatic marker. Both proteins co-localized at the nuclear periphery (B). Flueck C, Bartfai R, Niederwieser I, Witmer K, Alako BT, Moes S, Bozdech Z, Jenoe P, Stunnenberg HG, Voss TS. A major role for the Plasmodium falciparum ApiAP2 protein PfSIP2 in chromosome end biology. PLoS Pathog. 2010 6:e1000784.
See original on MMP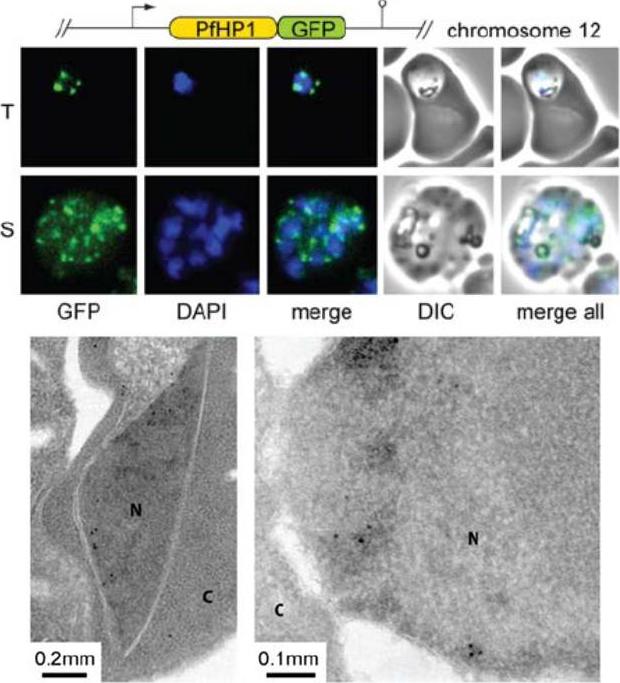
PfHP1 localizes to discrete regions at the nuclear periphery. Upper panel: PfHP1-GFP localization in unfixed 3D7/HP1-GFP parasites. T, trophozoites. S, schizonts. Lower panel: Localization of PfHP1-GFP by electron microsopy. Antibodies specific to GFP detect PfHP1-GFP at the nuclear periphery, reminiscent of the pattern seen by fluorescence microscopy (N, nucleus; C, cytoplasm). Schematic map of the transfection constructs is shown above the upper panel.Flueck C, Bartfai R, Volz J, Niederwieser I, Salcedo-Amaya AM, Alako BT, Ehlgen F, Ralph SA, Cowman AF, Bozdech Z, Stunnenberg HG, Voss TS. Plasmodium falciparum heterochromatin protein 1 marks genomic loci linked to phenotypic variation of exported virulence factors. PLoS Pathog. 2009 Sep;5(9):e1000569.
See original on MMP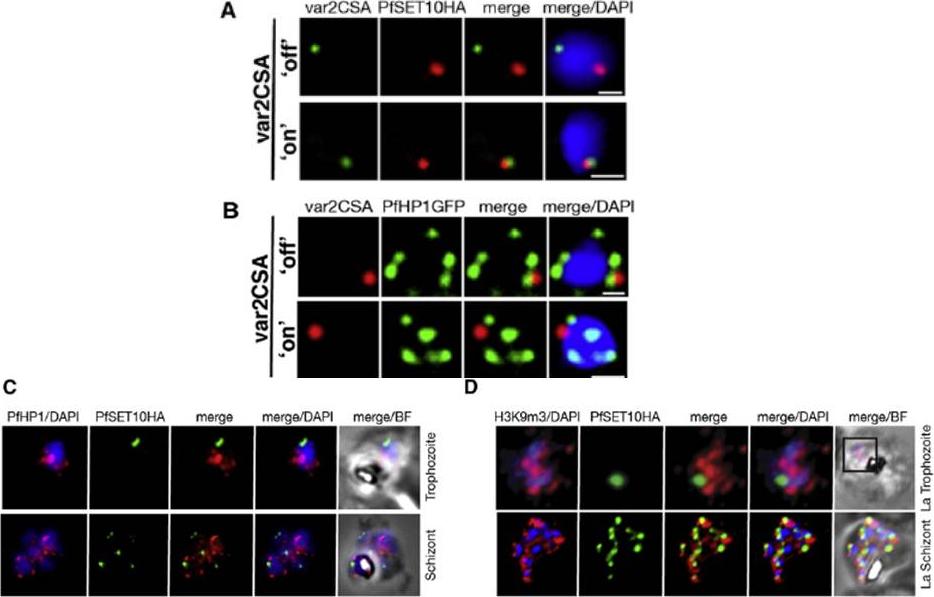
A. IFA-FISH of 3D7SET10-HA, in which var2csa (PFL0030c) activation has been selected by CSA adherence (‘‘on’’) (bottom panel). Unselected 3D7SET10-HA (top panel) has var2csa ‘‘off.’’ The localization of var2csa (green) and PfSET10 (red) is shown with both merged with the nucleus stained by DAPI (blue). Scale bars are equivalent to 0.5 mm. B. PfHP1 does not localize with active var2csa (‘‘on’’). The var2csa gene (red) location is shown with respect to PfHP1 protein (green). C. PfHP1 (red) and D. histone mark H3K9me3 (red,), known markers of silent var gene clusters and PfSET10 (green), do not colocalize in the nucleus stained with DAPI (blue).Volz JC, Bártfai R, Petter M, Langer C, Josling GA, Tsuboi T, Schwach F, Baum J, Rayner JC, Stunnenberg HG, Duffy MF, Cowman AF. PfSET10, a Plasmodium falciparum Methyltransferase, Maintains the Active var Gene in a Poised State during Parasite Division. Cell Host Microbe. 2012 11(1):7-18.
See original on MMP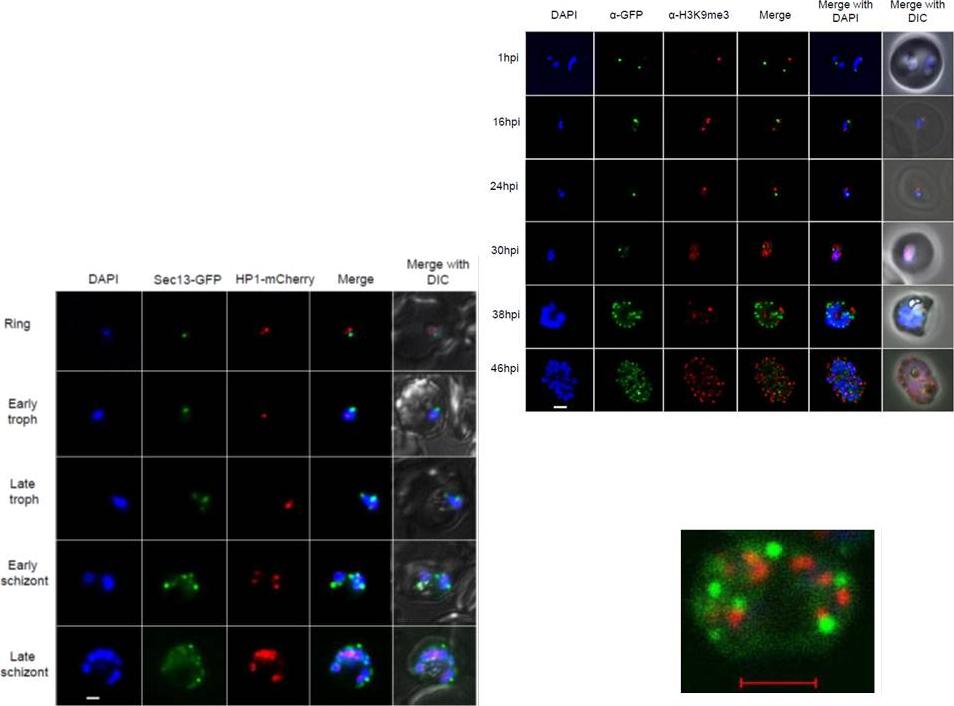
Right: PfSec13 does not co-localize with P. falciparum heterochromatin. IFAs of the PfSec13-GFP expressing parasites that were co-immunolabled with α-GFP (green) and α-H3K9me3 (red) at different stages of the IDC. In 85% of the counted nuclei of all stages (n=100), the H3K9me3 signal and PfSec13-GFP did not co-localize.Left: In vivo imaging of parasites expressing both PfSec13-GFP and PfHP1-HA-mCherry during the IDC. Although PfSec13 (green) and PfHP1 (red) localized to the nuclear periphery they do not co-localize.Righet lower corner: In vivo confocal microscopy of parasites expressing both PfSec13-GFP and PfHP1-HA-mCherry.Dahan-Pasternak N, Nasereddin A, Kolevzon N, Pe'er M, Wong W, Shinder V, Turnbull L, Whitchurch CB, Elbaum M, Gilberger TW, Yavin E, Baum J, Dzikowski R. PfSec13 is an unusual chromatin associated nucleoporin of Plasmodium falciparum, which is essential for parasite proliferation in human erythrocytes. J Cell Sci. 2013 126(Pt 14):3055-69 Reproduced with permission
See original on MMP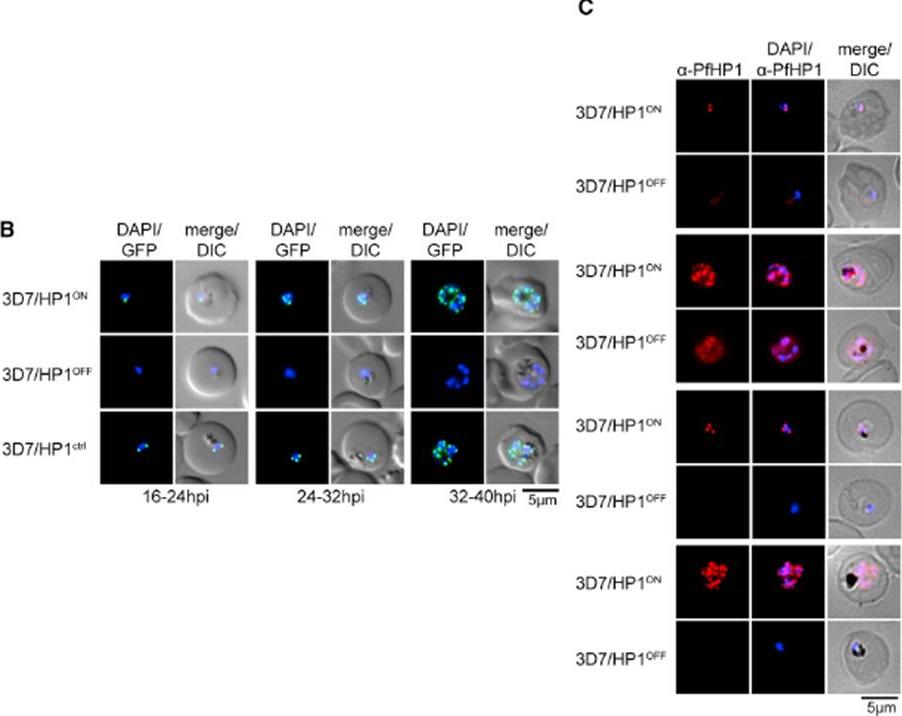
We applied the FKBP destabilization domain (DD) technique that allows modulating expression levels through the stabilizing compound Shield-1 and generated a clonal parasite line expressing endogenous PfHP1 as a C-terminally tagged GFP-DD fusion (3D7/HP1ON) (B) Expression and localization of PfHP1 in 3D7/HP1ON, 3D7/HP1OFF, and 3D7/HP1ctrl parasites by live fluorescence microscopy (images taken 12 hr after removal of Shield-1). Live-cell imaging revealed the expected perinuclear localization of tagged PfHP1 in 3D7/HP1ON and 3D7/HP1ctrl parasites throughout the IDC, whereas in 3D7/HP1OFF parasites PfHP1 was undetectable 12 hr after Shield-1 withdrawal. (C) Expression and localization of PfHP1 in 3D7/HP1ON and 3D7/HP1OFF parasites by IFA and western blot (Shield-1 removal at 4–12 hpi). After Shield-1 removal at 4–12 hpi, PfHP1 was still detectable but reduced in late ring stages (16–24 hpi) and early schizonts (32–40 hpi) and localized diffusely to the nucleoplasm and cytoplasm.Brancucci NM, Bertschi NL, Zhu L, Niederwieser I, Chin WH, Wampfler R, Freymond C, Rottmann M, Felger I, Bozdech Z, Voss TS. Heterochromatin protein 1 secures survival and transmission of malaria parasites. Cell Host Microbe. 2014 16(2):165-76.
See original on MMP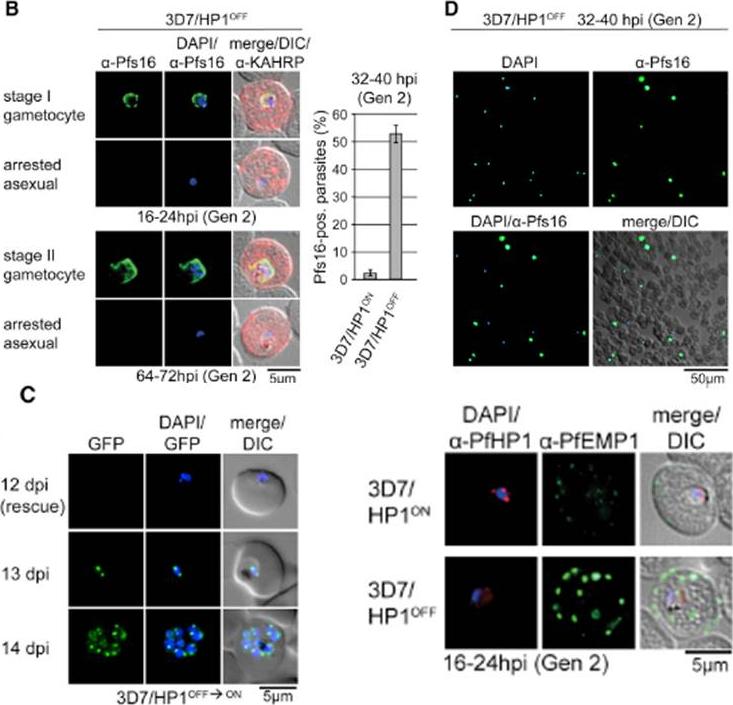
PfHP1 depletion induces gametocyte conversion. Distinction between 3D7/HP1OFF early gametocytes and arrested trophozoites by IFA (left) and proportion of Pfs16/KAHRP-positive parasites in 3D7/HP1ON and 3D7/HP1OFF (right). Values show the mean ± SD of three biological replicates (100 KAHRP-pos. iRBCs were scored per experiment). (D) a-Pfs16 IFA of a 3D7/HP1OFF parasite culture (Shield-1 removal at 4–12 hpi) at 32–40 hr postreinvasion (image taken at 40X magnification). The gametocyte hyperinduction phenotype is highlighted by the high proportion of Pfs16-positive stage I gametocytes among all DAPI-positive iRBCs. (C) Growth-arrested 3D7/HP1OFF parasites reenter mitotic proliferation after Shield-1 replenishment. dpi, days postreinvasion. PfHP1 depletion causes reversible cell-cycle arrest at the G1/S transition phase. Lower right panel: a-PfHP1/a-PfEMP1 (mAb 6H1) IFAs of 3D7/HP1ON and 3D7/HP1OFF parasites at 16–24 hpi in generation 2. PfEMP1 in 3D7/HP1OFF parasites at the single-cell level is hyperexpressed and correctly trafficked to the iRBC surfaceBrancucci NM, Bertschi NL, Zhu L, Niederwieser I, Chin WH, Wampfler R, Freymond C, Rottmann M, Felger I, Bozdech Z, Voss TS. Heterochromatin protein 1 secures survival and transmission of malaria parasites. Cell Host Microbe. 2014 16(2):165-76.
See original on MMP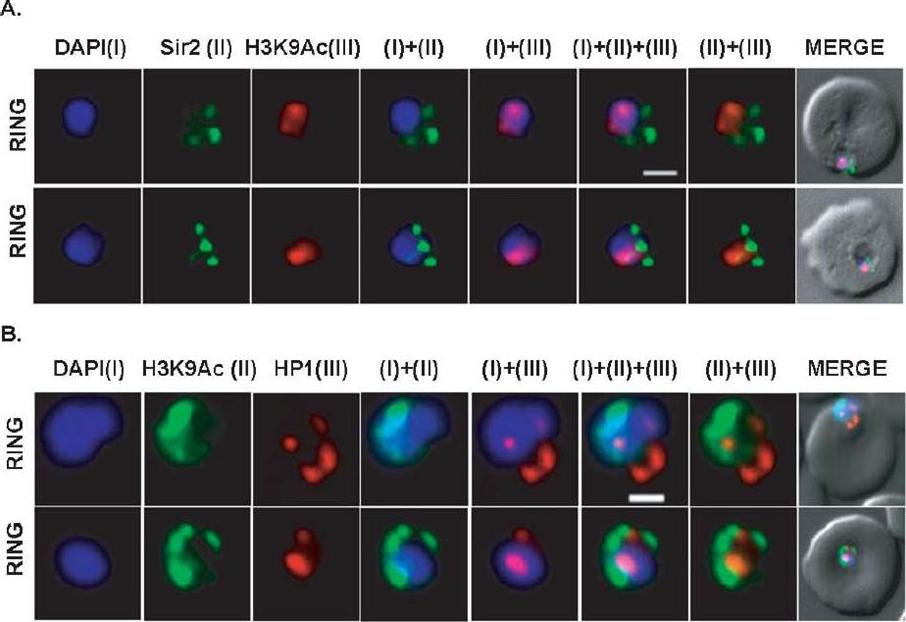
Differential localization patterns of H3K9Ac, PfSir2 and PfHP1. (A) Differential distribution of PfSir2 and H3K9Ac in Plasmodium falciparum. Dual Immuno-fluorescence analysis of PfSir2 (green) and H3K9Ac (red) reveals PfSir2 as sharp foci around nuclei and H3K9Ac as sharp foci closely attached to nuclei periphery. There is no colocalization of PfSir2 and H3K9Ac in ring stage. Scale bar measures 1 μM. DAPI stains nuclei. (B) Differential distribution of H3K9Ac (green) and PfHP1, specific for H3K9me3 repressive histone marks (red). Dual immuno-fluorescence assay shows no colocalization between these two proteins. Scale bar measures 1 μM. DAPI stains nuclei.Srivastava S, Bhowmick K, Chatterjee S, Basha J, Kundu TK, Dhar SK. Histone H3K9 acetylation level modulates gene expression and may affect parasite growth in human malaria parasite Plasmodium falciparum. FEBS J. 2014 Sep 22. [Epub ahead of print]
See original on MMP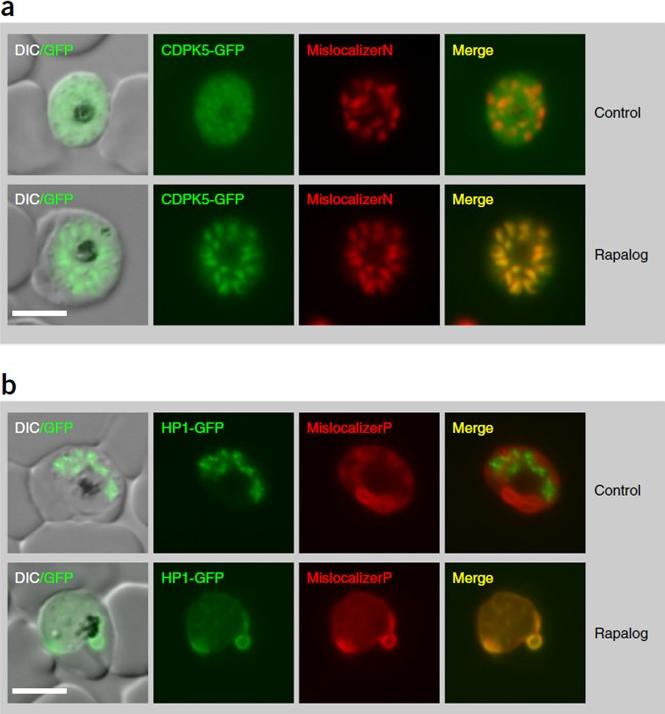
Localization and KS with protein targets. (a,b) Knockin cell lines of CDPK5-2×FKBP-GFP with an mCherry-tagged nuclear mislocalizer (MislocalizerN) (a) and HP1-2×FKBP-GFP with a plasma-membrane mislocalizer (MislocalizerP). In synchronized parasites, KS of CDPK5 caused an accumulation of segmented schizonts. (b). Representative fluorescence microscopy images of rapalog-treated and control cells (after 16 h). Mislocalization of HP1 caused an accumulation of gametocytes.Birnbaum J, Flemming S, Reichard N, Soares AB, Mesén-Ramírez P, Jonscher E, Bergmann B, Spielmann T. A genetic system to study Plasmodium falciparum protein function. Nat Methods. 2017 Mar 13.
See original on MMP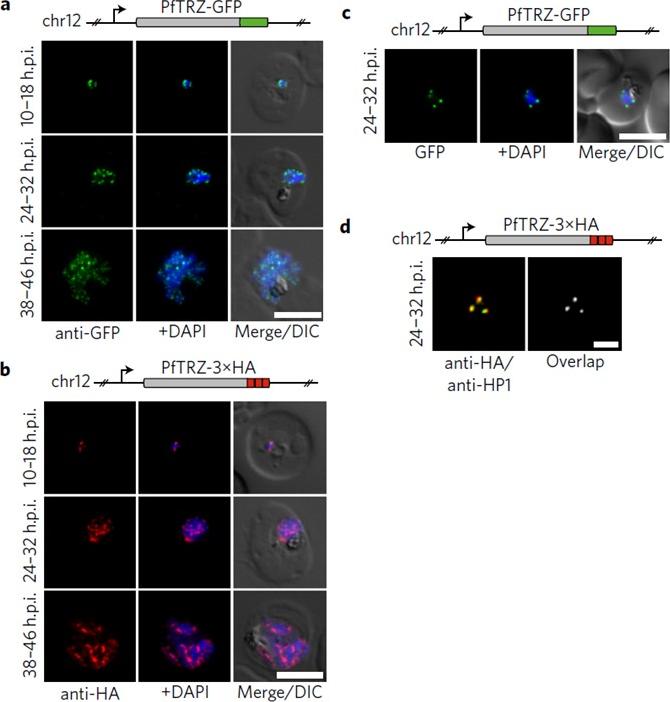
Genome-wide binding profile of PfTRZ. a,b, Localization of endogenous PfTRZ-GFP (a) and PfTRZ-3×HA (b), detected by IFA in three different IDC stages. h.p.i., hours post-invasion. Scale bars, 5 μm. Results are representative of three biological replicate experiments. c, Live imaging of endogenous PfTRZ-GFP. Scale bar, 5 μm. Results are representative of three biological replicate experiments. d, Co-localization of PfTRZ-3×HA and PfHP1 by confocal laser-scanning microscopy. The Pearson co-localization map visualizes overlapping signals (right). Scale bar, 2 μm. PfTRZ-GFP and PfTRZ-3×HA localized to distinct foci at the nuclear periphery throughout the intra-erythrocytic developmental cell cycle. The number of PfTRZ foci and total PfTRZ expression levels increased in schizonts (a,b). Confocal laser-scanning microscopy confirmed the exclusive co-localization of PfTRZ with PfHP1 at chromosome-end clusters.Bertschi NL, Toenhake CG, Zou A, Niederwieser I, Henderson R, Moes S, Jenoe P, Parkinson J, Bartfai R, Voss TS. Malaria parasites possess a telomere repeat-binding protein that shares ancestry with transcription factor IIIA. Nat Microbiol. 2017 2:17033. PMID: 28288093
See original on MMP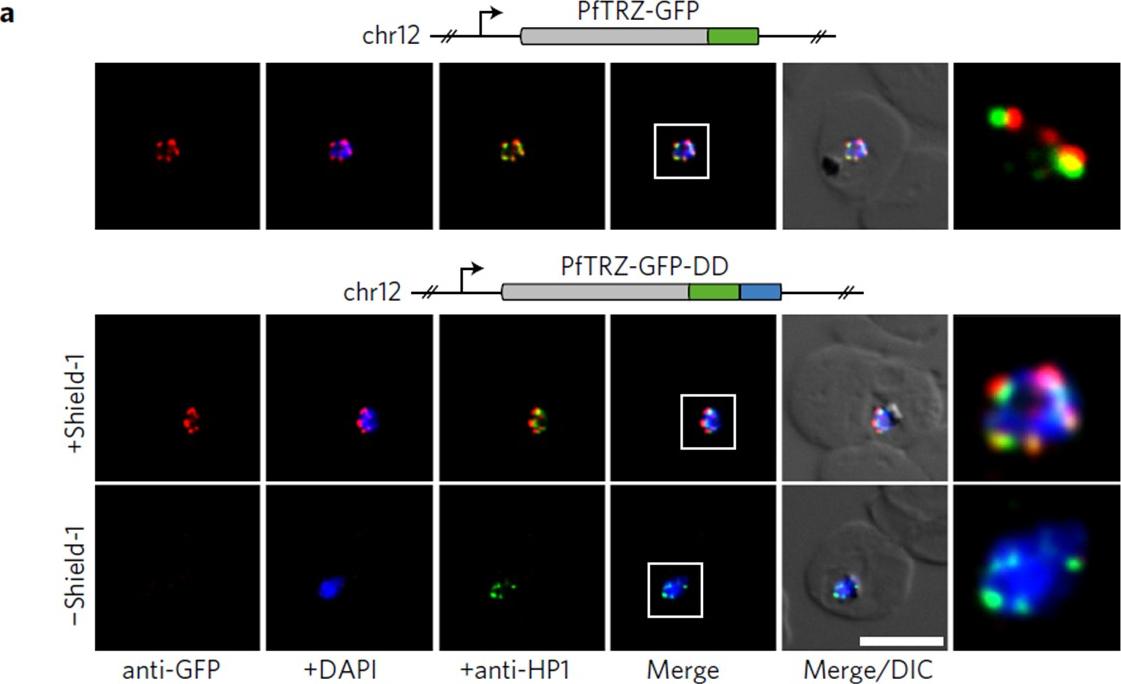
PfTRZ is required for proliferation and telomere length homeostasis. a, Co-localization of PfTRZ and PfHP1 in 3D7/TRZGFP, 3D7/TRZGFP-ON(+Shield-1) and 3D7/TRZGFP-OFF (−Shield-1) trophozoites. White frames refer to the magnified view in the rightmost images. Scale bar, 5 μm. Results are representative of three biological replicate experiments. PfTRZ-GFP-DD displayed the expected perinuclear expression pattern in 3D7/TRZGFP-ON parasites, but was barely detectable in parasites cultured in the absence of Shield-1 (3D7/TRZGFP-OFF).Bertschi NL, Toenhake CG, Zou A, Niederwieser I, Henderson R, Moes S, Jenoe P, Parkinson J, Bartfai R, Voss TS. Malaria parasites possess a telomere repeat-binding protein that shares ancestry with transcription factor IIIA. Nat Microbiol. 2017 2:17033.
See original on MMP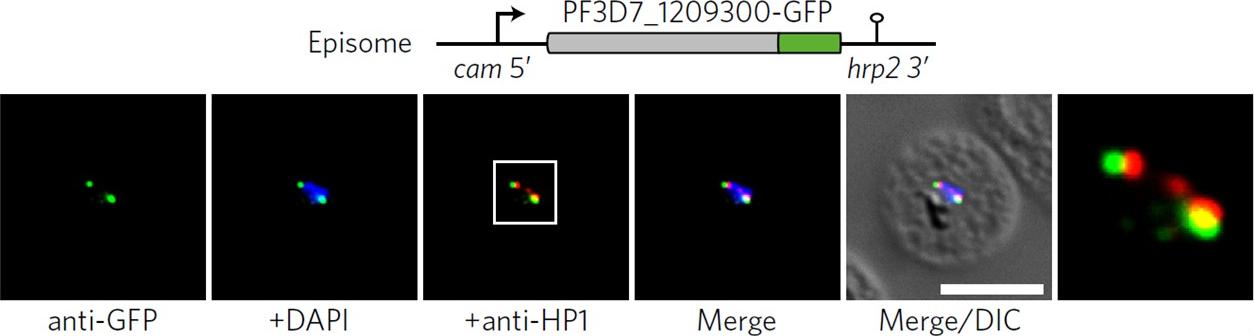
Co-localization of ectopic PF3D7_1209300-GFP and PfHP1 in trophozoites. The white frame refers to the magnified view in the rightmost image. DIC, differential interference contrast. Scale bar, 5 μm. Results are representative of three biological replicate experiments. IFAs demonstrated that an ectopically expressed green fluorescent protein (GFP)-tagged version of this factor localized to distinct spots at the nuclear periphery, in close proximity to the chromosome end marker PfHP1 (HP1, heterochromatin protein 1.Bertschi NL, Toenhake CG, Zou A, Niederwieser I, Henderson R, Moes S, Jenoe P, Parkinson J, Bartfai R, Voss TS. Malaria parasites possess a telomere repeat-binding protein that shares ancestry with transcription factor IIIA. Nat Microbiol. 2017 2:17033.
See original on MMPMore information
| PlasmoDB | PVX_123682 |
| GeneDB | PVX_123682 |
| Malaria Metabolic Pathways | Localisation images Pathways mapped to |
| Previous ID(s) | null |
| Orthologs | PBANKA_1436100 , PCHAS_1438100 , PF3D7_1220900 , PKNH_1440000 , PVP01_1439200 , PY17X_1438500 |
| Google Scholar | Search for all mentions of this gene |