PVX_114670 ER lumen protein retaining receptor, putative (ERD2)
Disruptability [+]
| Species | Disruptability | Reference | Submitter | |
|---|---|---|---|---|
| P. berghei ANKA |
Refractory |
PlasmoGEM (Barseq) | PlasmoGEM | |
| P. falciparum 3D7 |
Refractory |
USF piggyBac screen (Insert. mut.) | USF PiggyBac Screen | |
Mutant phenotypes [+]
None reported yet. Please press the '+' button above to add one.Imaging data (from Malaria Metabolic Pathways)
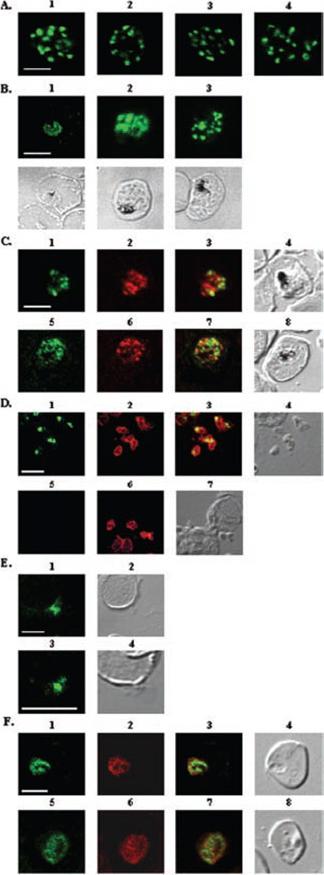
A, localization of RAMA in rhoptry organelles. Confocal microscopy on schizont stage parasites stained with rabbit anti-RAMA-B (1), anti-RAMA-C (2), anti-RAMA-D (3), or anti-RAMA-E (4) sera. B, localization of RAMA in immature parasites. Confocal microscopy on parasites stained with rabbit anti-RAMA-D serum: early trophozoite (1), late trophozoite (2), and early schizont (3) stages. Corresponding differential interference contrast (DIC) images are shown. C, trafficking of RAMA through the secretory pathway. Confocal microscopy on parasites stained with mouse anti-RAMA-E (1 and 5) and either anti-PfGRP (2) or anti-PfERD2 (6) rabbit antibodies. Corresponding overlay (3 and 7) and DIC (4 and 8) images are shown. D, localization of p60/RAMA in free merozoites. Confocal microscopy on parasites stained with mouse anti-MSP4 (2 and 6), and rabbit anti-RAMA-D (1) or anti-RAMA-B (5) sera. Corresponding overlay (3) and DIC (4 and 7) images are shown. E, discharge of RAMA from rhoptries. Confocal microscopy on CytB-treated merozoites incubated with RBCs, stained with rabbit anti-RAMA-D serum (1 and 3). Corresponding DIC images (2 and 4) are shown. F, association with the PV. Confocal microscopy on early ring stage-parasites stained with rabbit anti-RAMA-E serum (1 and 5) and monoclonal anti-RAP1 (2 and 6) antibodies. Corresponding overlay (3 and 7) and DIC (4 and 8) images are shown. Bars in 1 represent 5 mm. RAMA is synthesized as a 170-kDa protein in early trophozoites, several hours before rhoptry formation and is transiently localized within the endoplasmic reticulum and Golgi within lipid-rich microdomains.Topolska AE, Lidgett A, Truman D, Fujioka H, Coppel RL. Characterization of a membrane-associated rhoptry protein of Plasmodium falciparum. J Biol Chem. 2004 279:4648-4656.
See original on MMP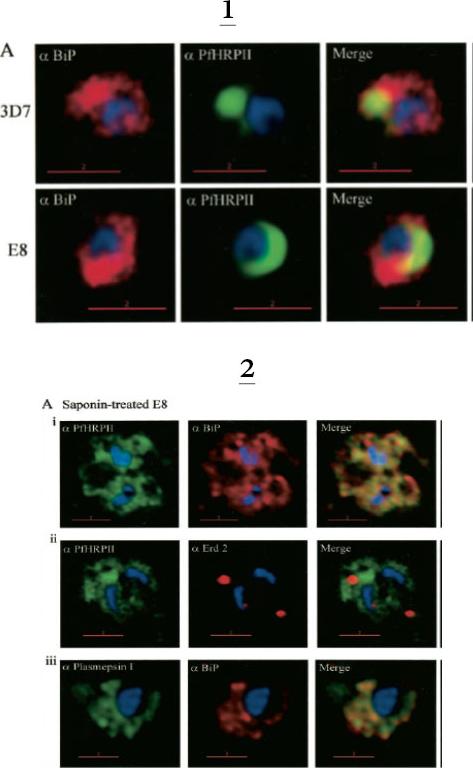
1. Comparative analysis of secretory recruitment of HRPII, export of parasite proteins in E8- and 3D7-infected erythrocytes, and distribution of total PfHRPII, PfHRPIImyc, plasmepsin I, and BiP in parasites and isolate food vacuoles. A, ring-infected red cells from E8 and 3D7-strains were treated with brefeldin A, stained for PfBiP (red), PfHRPII (green), and Hoechst dye (blue) in indirect immunofluorescence assays, and imaged using DeltaVision. Single optical sections showing a merge of red and green as yellow are shown. In the presence of BFA, all cell-associated HRPIIs detected in both E8 and 3D7 reside within regions that stain with the ER marker PfBiP, confirming that HRPII is recruited to the secretory pathway.2. Membrane association of parasite-HRPII in E8 and ACPGFP cells. A, 30–33-h trophozoite-infected red cells from 3D7 and E8 strains were attached to poly-L-lysine-coated cover slips and then permeabilized with 0.01% saponin to release the erythrocytic contents. The adherent cells were then fixed with formaldehyde and probed for the distribution of the indicated proteins by indirect immunofluorescence microscopy. HRPII and plasmepsin I show substantial colocalization with PfBiP in E8 cells but not markers of the Golgi (ERD2).Akompong T, Kadekoppala M, Harrison T, Oksman A, Goldberg DE, Fujioka H, Samuel BU, Sullivan D, Haldar K. Trans expression of a Plasmodium falciparum histidine-rich protein II (HRPII) reveals sorting of soluble proteins in the periphery of the host erythrocyte and disrupts transport to the malarial food vacuole. J Biol Chem. 2002 277:28923-33.
See original on MMP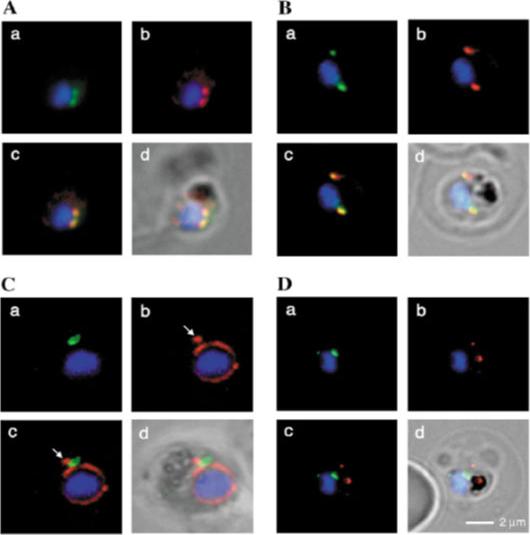
Fluorescence microscopy on fixed parasites. (A) PfGRASP-GFP colocalises with antiPfGRASP-specific antibodies. PfGRASP-GFP is tightly confined to two compartments (a, green) near the parasite nucleus (a, blue). AntiPfGRASP-specific antibodies show a similar staining pattern (b, red with nucleus in blue). Merged image shows identical colocalisation. (B) PfGRASP-GFP colocalises with the cis-Golgi marker ERD2. PfGRASP-GFP (a, green) accumulates in two discrete compartments in close proximity to the nucleus (a, blue). Anti-PfERD2 antibodies recognize similar structures (b, red with nucleus in blue). Merged image shows colocalisation of compartments (c, yellow). (C) PfGRASP-GFP defines a compartment that is distinct from the ER. At the early stages of the parasite life cycle (<16 hours post invasion) PfGRASP is restricted to one compartment (a, green) juxtapose to the nucleus (a, blue). The ER is visualised by anti-PfBiP-specific antibodies (b, red). The membranous system of the ER forms an envelope around the nucleus (b, blue) with one protrusion (indicated by arrow). Merged image shows no colocalisation of the two compartments (c). (D) PfGRASP-GFP does not colocalise with the trans-Golgi marker PfRab6. PfGRASP accumulates in two discrete foci (a, green) adjacent to the nucleus (a, blue). Antibodies against PfRab6 visualise two distinct sites within the parasite (b, red with nucleus in blue). Merged image shows no colocalisation of the PfGRASP defined compartment with PfRab6 (c).Struck NS, de Souza Dias S, Langer C, Marti M, Pearce JA, Cowman AF, Gilberger TW. Re-defining the Golgi complex in Plasmodium falciparum using the novel Golgi marker PfGRASP. J Cell Sci. 2005 118(Pt 23):5603-13.
See original on MMP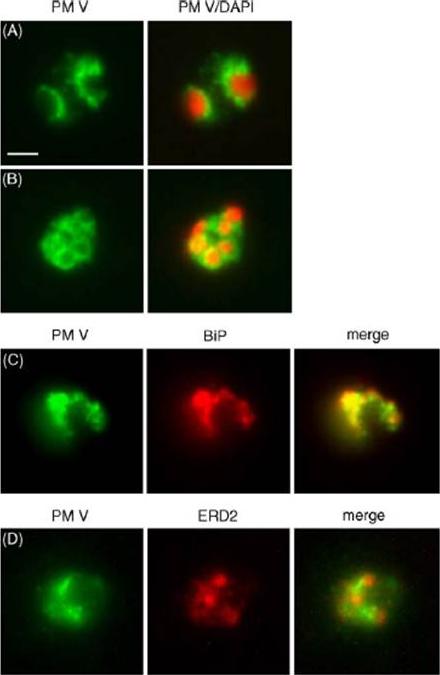
Localization of plasmepsin V (PM V). Indirect immunofluorescence assays of PM V (green) in trophozoites (A) and a ~6N schizont (B) in formaldehyde-fixed blood smears. Nuclei were visualized with DAPI (pseudocolored red). (C, D) Colocalization of PM V and the ER protein BiP (C) or the Golgi marker ERD2 (D) in trophozoites. Bar, 2 mm. In trophozoites and schizonts, PM V was concentrated around the nucleus with lower levels dispersed in the surrounding cytoplasm (Fig. 5A and B). These patterns of fluorescence suggest that PM V resides in the endoplasmic reticulum (ER), which in P. falciparum has been shown to include the nuclear envelope.Klemba M, Goldberg DE. Characterization of plasmepsin V, a membrane-bound aspartic protease homolog in the endoplasmic reticulum of Plasmodium falciparum. Mol Biochem Parasitol. 2005 143:183-191. Copyright Elsevier 2010.
See original on MMP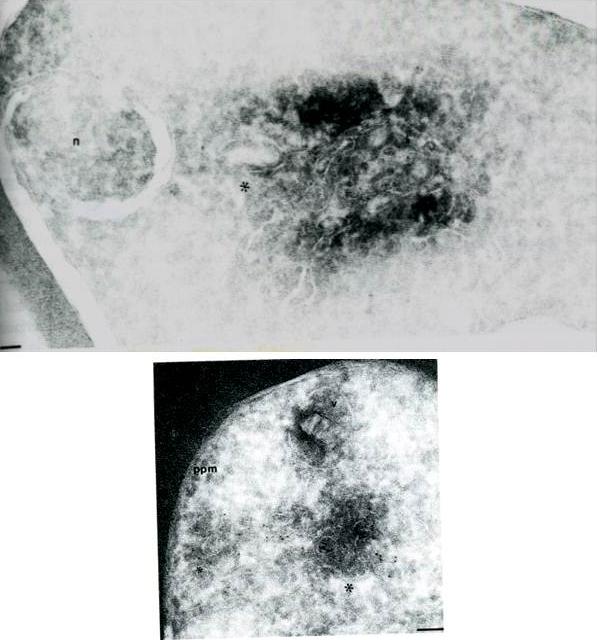
Cryosections of ring/trophozoite-infected Erythrocytes were probed with anti-Pf ERD2 (A) antibodies from non-immune sera (B) or anti-Pfrab6 (C) and goat anit-rabbit antibodies conjugated to 5 nm gold particles. In panel A, labeling of PfERD2 is detected in a perinuclear, tubulovesicular structure (*) with 1:100 anti PfERD2. The nucleus is marked by a n. Bar indicates 0.08 mm. In panel B, labeling of Pfrab6 (1:1OO) is seen in tubulovesicular clusters in peripheral regions of the cell. One cluster (large *) is proximal to the digestive food vacuole (marked with v). A second tubulovesicular cluster (small *) lies next to the parasite plasma membrane (marked by ppm). Bar indicates 0.1 mm. Van Wye J, Ghori N, Webster P, Mitschler RR, Elmendorf HG, Haldar K. Identification and localization of rab6, separation of rab6 from ERD2 and implications for an 'unstacked' Golgi, in Plasmodium falciparum. Mol Biochem Parasitol. 1996 83:107-20. Copyright Elsevier 2010
See original on MMP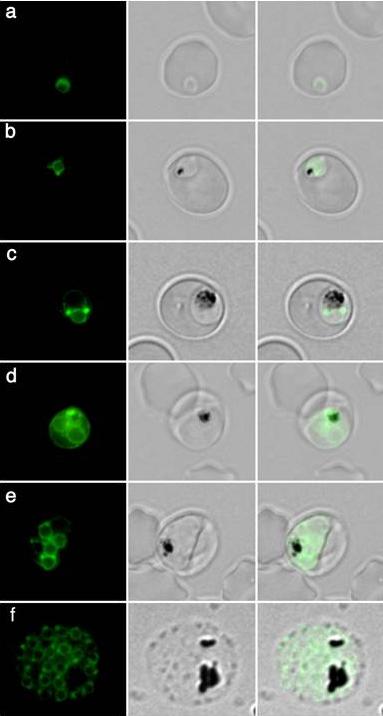
Live transgenic parasites expressing PfERD2-GFP were imaged by fluorescence microscopy. In early ring stages (8 hours post invasion, a) a weak crescent-shaped fluorescence is observed. At 16-24 hours post invasion two protrusions of intense PfERD2-GFP fluorescence are connected with weaker perinuclear staining (b-c). By 32-40 hours post invasion ongoing schizogony is observed. Nuclear division is indicated by additional, multiple perinuclear distributions of PfERD2-GFP (d-e). At 48 hours post invasion the late schizont stage is reached. Note that every forming merozoite displays one focused PfERD2-GFP signal (f).Struck NS, de Souza Dias S, Langer C, Marti M, Pearce JA, Cowman AF, Gilberger TW. Re-defining the Golgi complex in Plasmodium falciparum using the novel Golgi marker PfGRASP. J Cell Sci. 2005 118:5603-13
See original on MMP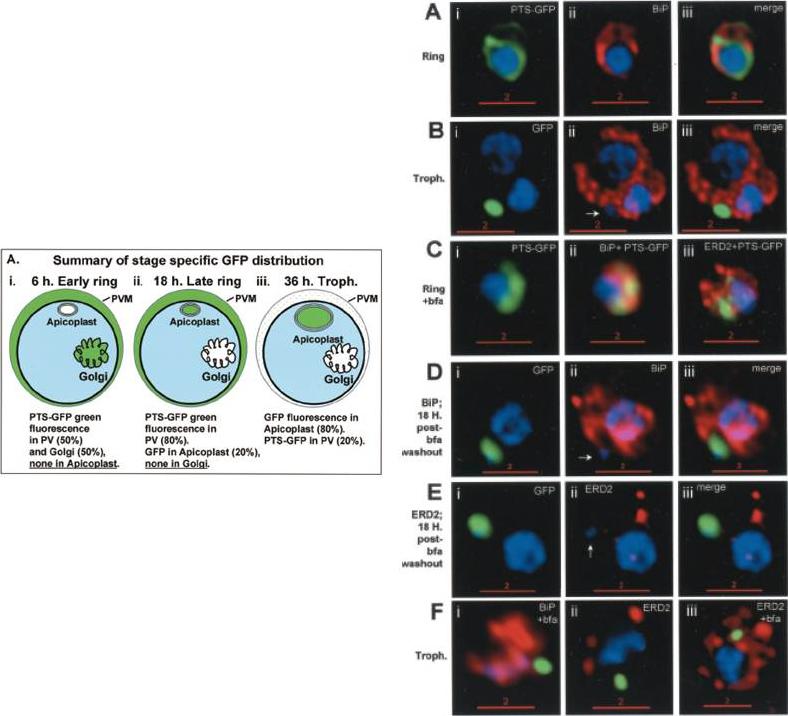
Stage-specific localization of apicoplast-targeted GFP and the effects of brefeldin A (bfa). Distribution of green fluorescence and PfBiP (red) in a early-ring (A, i–iii) and a trophozoite (B, i–iii; a late, 33 h, trophozoite with two nuclei stained in blue is shown). Distribution of green fluorescence and indicated secretory marker (red) in: rings incubated with Bfa for 24 h (C, i–iii); rings incubated with Bfa for 24 h, washed, and grown for 18 h in absence of drug (D, i–iii; E, i–iii); and trophozoites incubated with or without Bfa (F, i–iii). Blue indicates DNA stained with Hoechst 33342. White arrows in B, ii, D, ii, and E, ii, indicate apicoplast DNA. Scale bars as indicated in microns.Cheresh P, Harrison T, Fujioka H, Haldar K. Targeting the malarial plastid via the parasitophorous vacuole. J Biol Chem. 2002 277:16265-77.
See original on MMP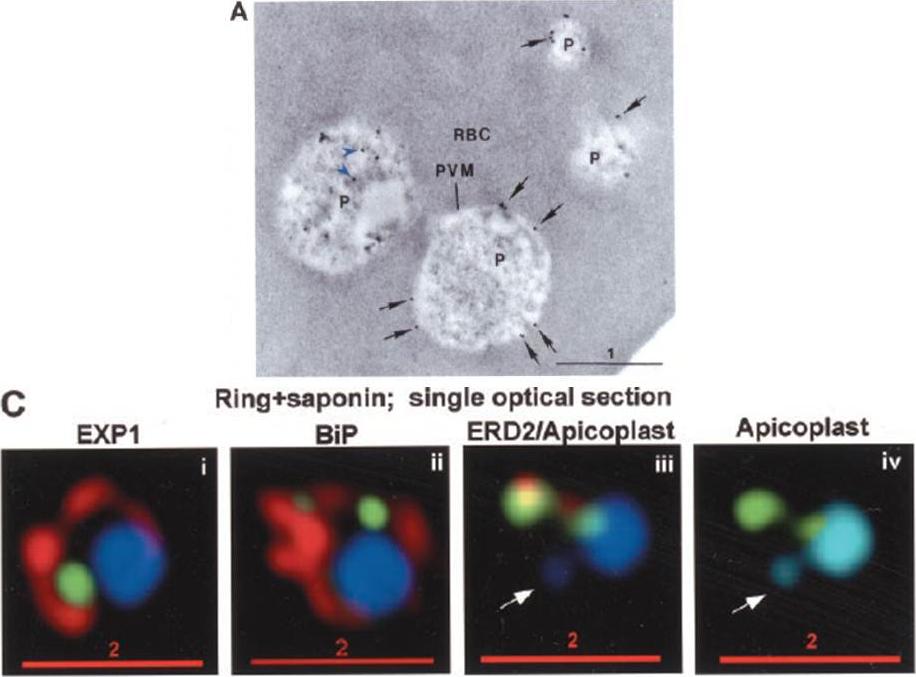
PTS-GFP is detected in the parasitophorous vacuole and in apposition with the Golgi in early rings. A, immunoelectron micrograph of rings showing localization of PTS (plastid-targeting-signal)-GFP in the parasitophorous vacuole and PVM (black arrows) as well as within the parasite (P; blue arrowheads). RBC, red blood cell. Scale bar, 1 mm.. C, i–iv: single optical sections showing green fluorescence in young rings permeabilized with 0.01% saponin relative to secretory markers PfEXP1, PfBiP, PfERD2 (shown in red in i–iii), and apicoplast DNA (marked with an arrow in iii and iv), as detected by indirect immunofluorescence and DeltaVision Microscopy. In C, iv, the Hoechst stain is pseudo-colored cyan to facilitate visualization of apicoplast DNA.Cheresh P, Harrison T, Fujioka H, Haldar K. Targeting the malarial plastid via the parasitophorous vacuole. J Biol Chem. 2002 277:16265-77.
See original on MMP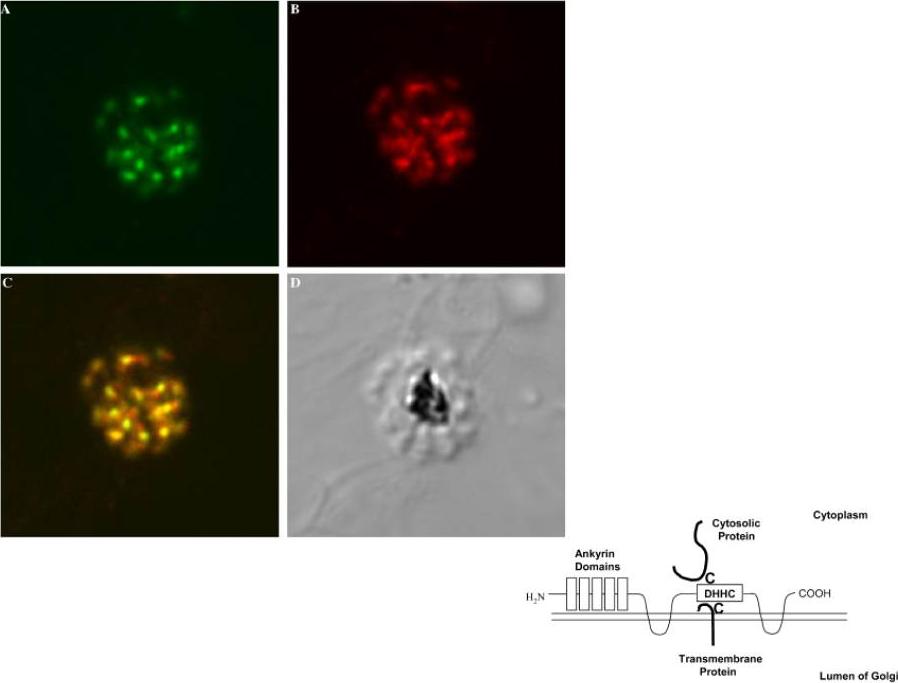
Left: Confocal microscopy localizes PfAnkDHHC (palmitoyl transferase) to Golgi. 3D7 schizonts were labeled with (A) rat anti-PfAnkDHHC, and (B) rabbit anti-ERD2, a known Golgi marker. (C) Merged image of the two stains shows extensive co-localization. (D) Corresponding DIC image. All images 630x.Right: Proposed model of topology of PfAnkDHHC in the Golgi membrane. The DHHC domain is exposed to the cytosol to serve as a palmitoyl transferase to cytosoloic proteins or transmembrane proteins with cysteines.Seydel KB, Gaur D, Aravind L, Subramanian G, Miller LH. Plasmodium falciparum: characterization of a late asexual stage golgi protein containing both ankyrin and DHHC domains. Exp Parasitol. 2005 110:389-93. Copyright Elsevier 2010.
See original on MMP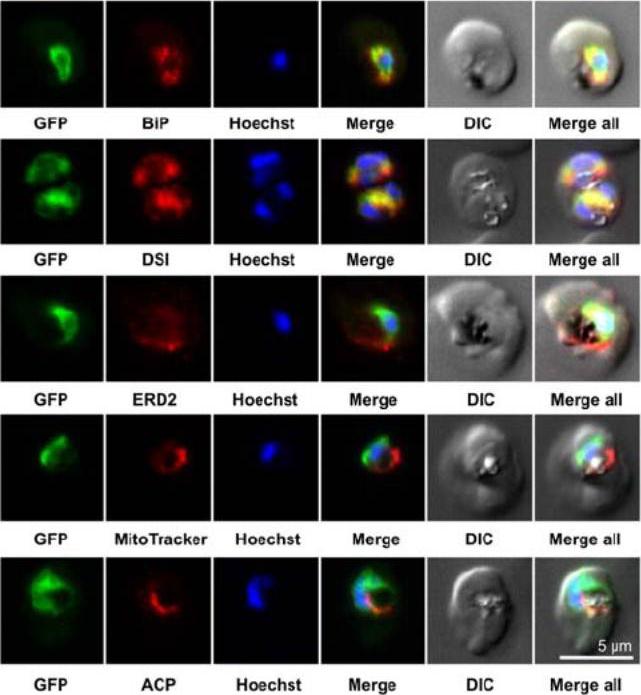
Colocalization studies. PfSCD-GFP was colocalized with different compartment markers: ER (PfBip, PfDSI), cis-Golgi (PfERD2), mitochondria (MitoTracker Red), and apicoplast (PfACP PFB0385w). PfSCD-GFP colocalized mostly with PfBiP and PfDSI (disulfide isomerase), two soluble ER markers, therefore confirming the ER localization of PfSCD-GFP. In contrast, the GFP-tagged PfSCD protein did not colocalize with ERD2, a known marker of the cis-Golgi.Gratraud P, Huws E, Falkard B, Adjalley S, Fidock DA, Berry L, Jacobs WR Jr, Baird MS, Vial H, Kremer L. Oleic acid biosynthesis in Plasmodium falciparum: characterization of the stearoyl-CoA desaturase and investigation as a potential therapeutic target. PLoS One. 2009 4(9):e6889.
See original on MMP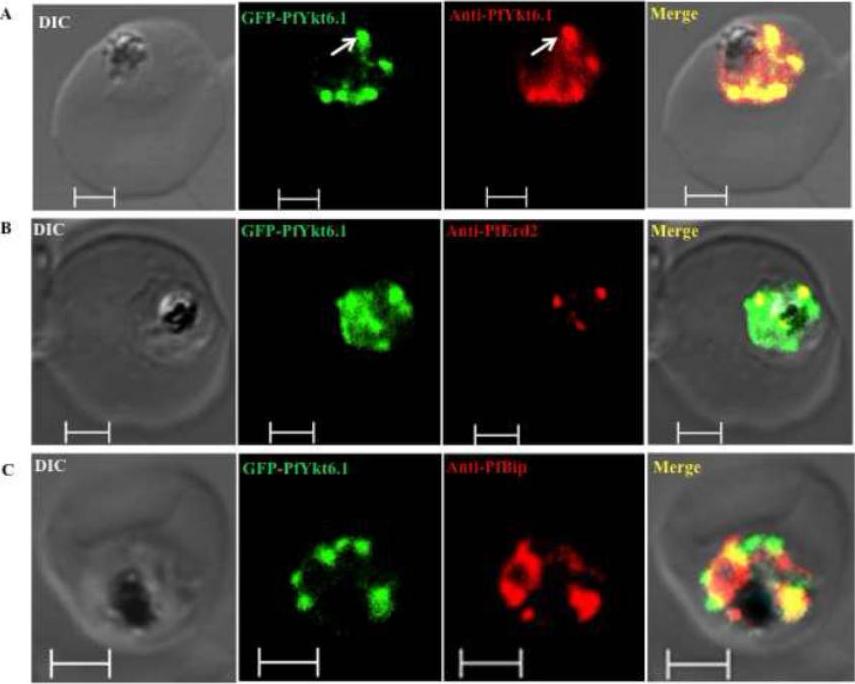
Compartmentalization of PfYkt6.1 in P. falciparum parasites. Trophozoite stage parasites exhibiting increased targeting of the PfYkt6.1 protein to organellar compartments were by confocal immunofluorescence microscopy using various organellar markers. (A) Immunofluorescence micrograph showing complete overlap between the GFP fluorescence and anti-PfYkt6.1 antibody signal in GFP-PfYkt6.1 expressing parasites. The GFP-PfYkt6.1 compartments were only partially labeled with antibodies against the Golgi marker PfErd2 (B), or the ER marker PfBip (C). Scale bars, 2μM. PfYkt6.1 appeared to be partly cytosolic and partly membrane-associated inside the parasite cytoplasm,Ayong L, Dasilva T, Mauser J, Allen CM, Chakrabarti D. Evidence for Prenylation-Dependent Targeting of a Ykt6 SNARE in Plasmodium falciparum. Mol Biochem Parasitol. 2010 175(2):162-8. Copyright Elsevier 2011.
See original on MMP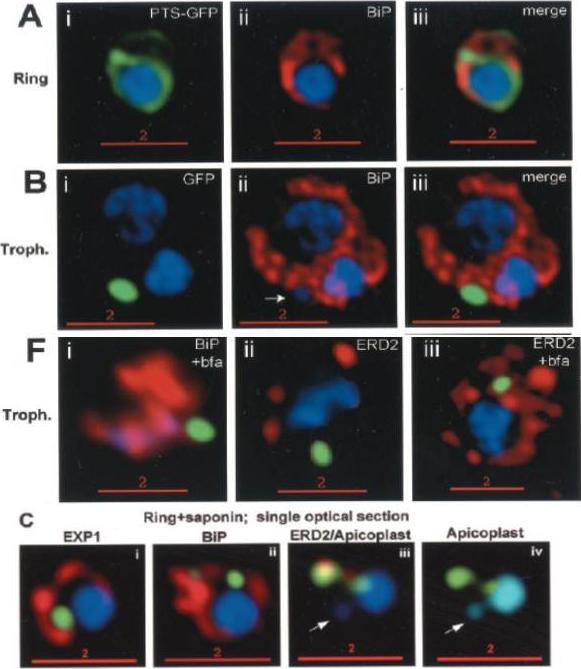
Stage-specific localization of apicoplast-targeted GFP. Distribution of green fluorescence and PfBiP (red) in a early-ring (A, i–iii) and a trophozoite (B, i–iii; a late, ~33 h, trophozoite with two nuclei stained in blue is shown). Distribution of green fluorescence and indicated secretory marker (red) in trophozoites incubated. Blue indicates DNA stained with Hoechst 33342. White arrows in B, ii, D, ii, and E, ii, indicate apicoplast DNA. Scale bars as indicated in microns. C, i–iv: single optical sections showing green fluorescence in young rings permeabilized with 0.01% saponin relative to secretory markers PfEXP1, PfBiP, PfERD2 (shown in red in i–iii), and apicoplast DNA (marked with an arrow in iii and iv), as detected by indirect. In C, iv, the Hoechst stain is pseudo-colored cyan to facilitate visualization of apicoplast DNA.Cheresh P, Harrison T, Fujioka H, Haldar K. Targeting the malarial plastid via the parasitophorous vacuole. J Biol Chem. 2002 277:16265-77.
See original on MMP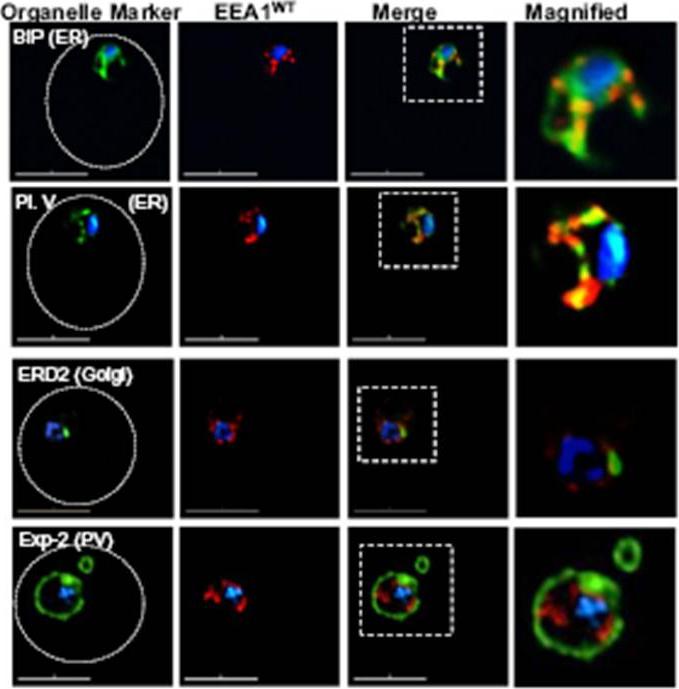
Localization of EEA1WT-mCherry as a marker for PI(3)P (phosphatidylinositol-3-phosphate) in the parasite ER. EEA1 = early endosomal antigen 1. Transgenic parasites transfected with EEA1 were fixed, permeabilized and probed with anti-mCherry as well as antibodies to endogenous P. falciparum markers (green) of the ER, like BIP (top row) and plasmepsin V (Pl. V, second row); a marker of the Golgi (ERD2, third row) or the PVM (Exp-2, bottom row). EEA1WT (red) is seen in punctuate ‘spots’ within the ER. Dotted circles show location of red cell membrane. Dotted squares show regions magnified in the right panels. Parasite nuclei were stained with Hoechst 33342 (blue). Scale bars, 5 mm. The overlap between markers and EEA1 indicates the presence of PI(3)P in the respective regions.Bhattacharjee S, Stahelin RV, Speicher KD, Speicher DW, Haldar K. Endoplasmic Reticulum PI(3)P Lipid Binding Targets Malaria Proteins to the Host Cell. Cell. 2012 148(1-2):201-12.
See original on MMP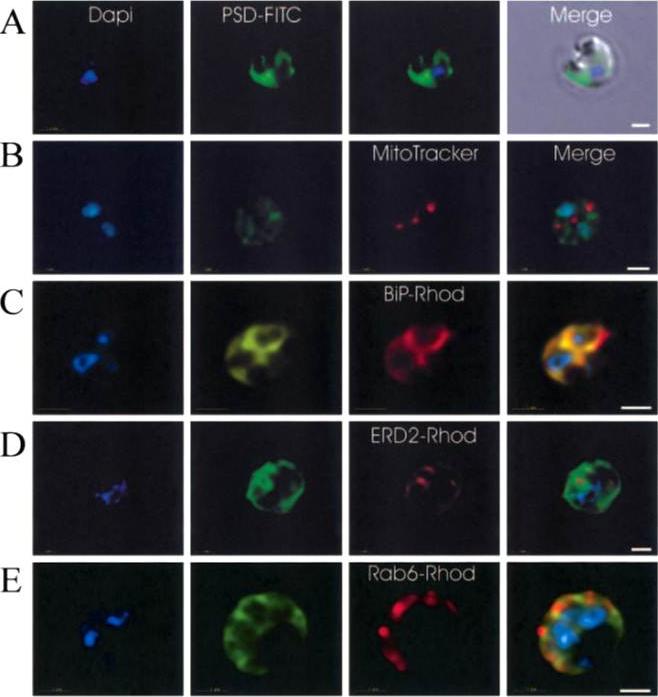
Immunofluorescence localization of PfPSD within P. falciparum-infected erythrocytes. A. FITC (which labels PfPSD) and DAPI (which labels nuclei) images were merged with the Nomarsky image to show the location of PSD labelling in the parasite. DAPI and FITC images were merged with the rhodamine channel corresponding to MitoTracker that labels the mitochondrion (B), BiP-rhodamine that labels the ER (C), ERD2-rhodamine that labels the cis-Golgi (D) or Rab6 -rhodamine that labels the trans-Golgi (E). All the images except (A) correspond to one selected z-section image after digital deconvolution. The bar corresponds to 1 mm. The entire PfPSD labelling was clearly co-localized with the BiP endoplasmic reticulum marker.Baunaure F, Eldin P, Cathiard AM, Vial H. characterization of a non-mitochondrial type I phosphatidylserine decarboxylase in Plasmodium falciparum. Mol Microbiol. 2004 51:33-46. Copyright John Wiley & Sons Ltd. 2010.
See original on MMP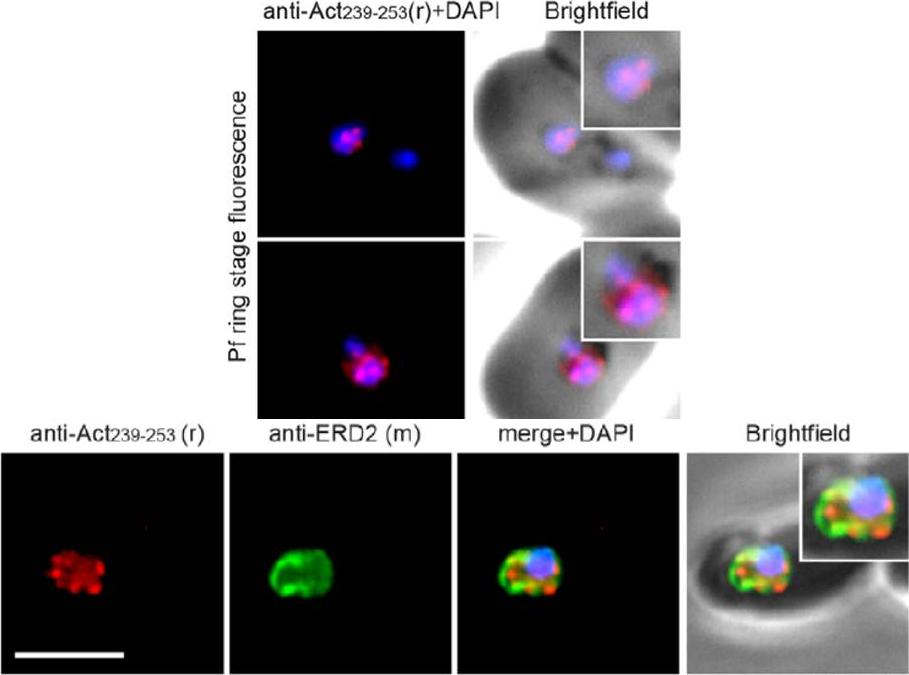
Upper panel: Widefield IFA of P. falciparum rings labelled with rabbit anti-Act239–253 (Red) and DAPI (Blue). Early ring stage asexual parasites were labelled with anti-actin and the nuclear marker DAPI. Very early rings demonstrated a consistent punctate labelling of actin within DAPI staining of the nucleus.Lower panel: Two colour widefield IFA using rabbit anti-Act239–253 (Red), rat anti-ERD2 (Green) and DAPI (Blue) Scale bar = 5 mm. The concentration of stabilised actin filaments at the nuclear periphery was confirmed using ERD2, a cis-Golgi marker that localises to defined sites adjacent to the nucleusAngrisano F, Riglar DT, Sturm A, Volz JC, Delves MJ, Zuccala ES, Turnbull L, Dekiwadia C, Olshina MA, Marapana DS, Wong W, Mollard V, Bradin CH, Tonkin CJ, Gunning PW, Ralph SA, Whitchurch CB, Sinden RE, Cowman AF, McFadden GI, Baum J. Spatial Localisation of Actin Filaments across Developmental Stages of the Malaria Parasite. PLoS One. 2012;7(2):e32188.
See original on MMP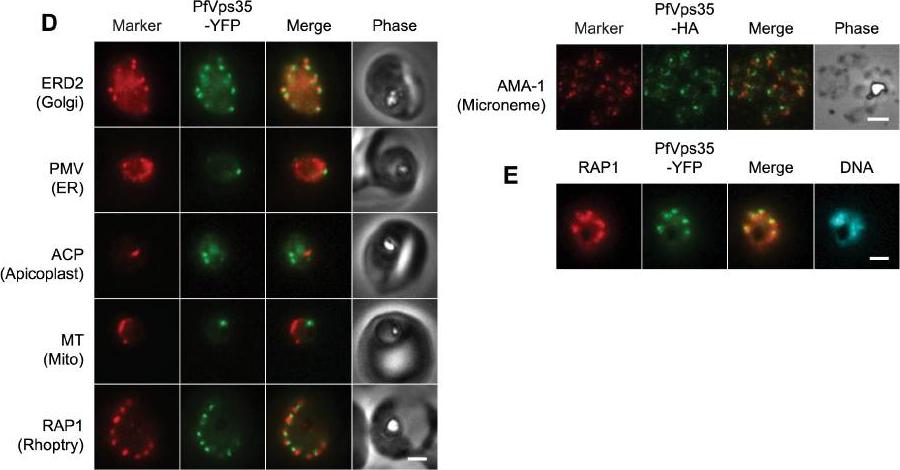
(D) Co-localization of PfVps35-YFP or PfVps35-HA and organellar markers in fixed parasites (except for MitoTracker, which was imaged live). PMV, plasmepsin V; ACP, acyl carrier protein; MT, MitoTracker Red CM-H2Xros; RAP1, rhoptry associated protein 1; AMA1, apical membrane antigen 1. The AMA1 panel shows free merozoites; all others are intraerythrocytic. Organelles labeled by the markers are indicated in parenthesis. Marker-derived fluorescence is pseudocolored red. (E) PfVps35-YFP is adjacent to developing rhoptries in a 2N parasite. Hoechst 33342 fluorescence (DNA) is pseudocolored cyan. In all panels, YFP fluorescence is pseudocolored green. Scale bars, 2 mm.Krai P, Dalal S, Klemba M. Evidence for a Golgi-to-Endosome Protein Sorting Pathway in Plasmodium falciparum. PLoS One. 2014 9(2):e89771.
See original on MMP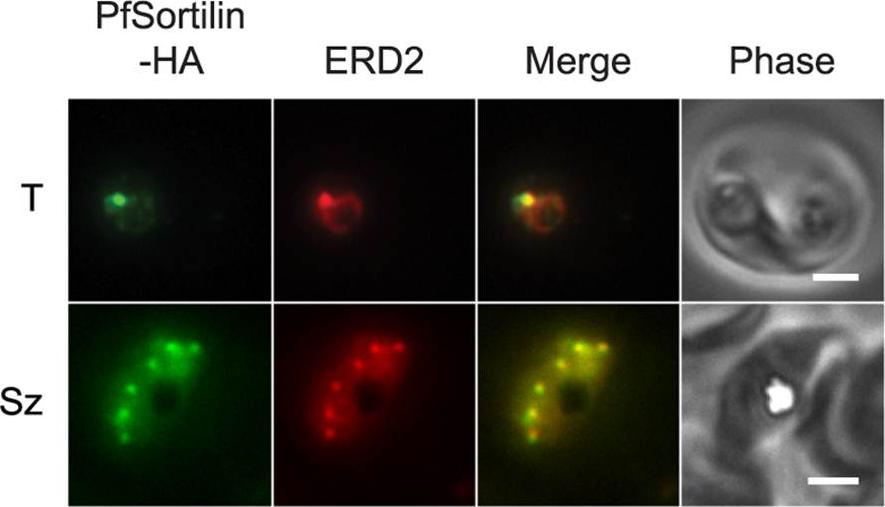
The putative protein sorting receptor PfSortilin localizes to the P. falciparum Golgi apparatus. Co-localization of PfSortilin-HA and ERD2 in aldehyde-fixed clone C9 parasites. T, trophozoite; Sz, schizont. Scale bar, 2 mm. In trophozoite- and schizont-stage parasites, PfSortilin-HA localized to puncta that were also recognized by antibodies to the Golgi marker ERD2 (Fig. 4C). In ring-stage parasites, PfSortilin-HA could not be detected above non-specific background, even though the Golgi apparatus was present as determined by ERD2 immunolocalization.Krai P, Dalal S, Klemba M. Evidence for a Golgi-to-Endosome Protein Sorting Pathway in Plasmodium falciparum. PLoS One. 2014 9(2):e89771. PMID:
See original on MMP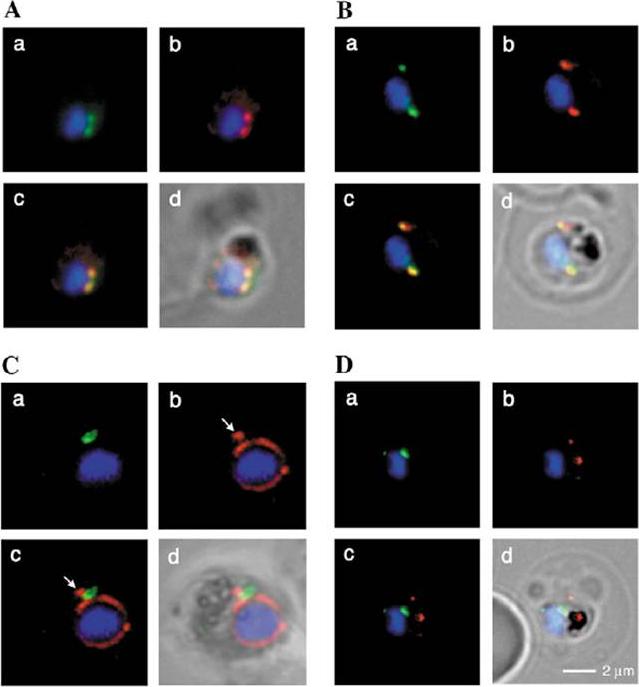
Spatial organisation of the PfGRASP-GFP defined compartment by fluorescence microscopy on fixed parasites. (A) PfGRASP-GFP colocalises with anti PfGRASP-specific antibodies. PfGRASP-GFP is tightly confined to two compartments (a, green) near theparasite nucleus (a, blue). AntiPfGRASP-specificantibodies show a similar staining pattern (b, red withnucleus in blue). Merged image shows the colocalisation of the compartments defined by either PfGRASP-specific antibodies or PfGRASP-GFP expressingparasites (c, yellow). (B) PfGRASP-GFP colocalises with the cis-Golgi marker ERD2. PfGRASP-GFP (a, green) accumulates in two discrete compartments in close proximity to the nucleus (a, blue). Anti-PfERD2 antibodies recognize similar structures (b, red with nucleus in blue). Merged image shows colocalisation of compartments (c, yellow). (C) PfGRASP-GFP defines a compartment that is distinct from the ER . At the early stages of the parasite life cycle (<16 hours post invasion) PfGRASP is restricted to one compartment (a, green) juxtapose to the nucleus (a, blue). The ER is visualised by anti-PfBiP-specific antibodies (b, red). The membranous system of the ER forms an envelope around the nucleus (b, blue) with one protrusion (indicated by arrow). Merged image shows no colocalisation of the two compartments (c). (D) PfGRASP-GFP does not colocalise with the trans-Golgi marker PfRab6. PfGRASP accumulates in two discrete foci (a, green) adjacent to the nucleus (a, blue). Antibodies against PfRab6 visualise two distinct sites within the parasite (b, red with nucleus in blue). Merged image shows no colocalisation of the PfGRASP defined compartment with PfRab6 (c) All panels labelled d in A-D are merges of fluorescent and bright-field images. Bar, 2 mm. Struck NS, de Souza Dias S, Langer C, Marti M, Pearce JA, Cowman AF, Gilberger TW. Re-defining the Golgi complex in Plasmodium falciparum using the novel Golgi marker PfGRASP. J Cell Sci. 2005 118(Pt 23):5603-13.
See original on MMP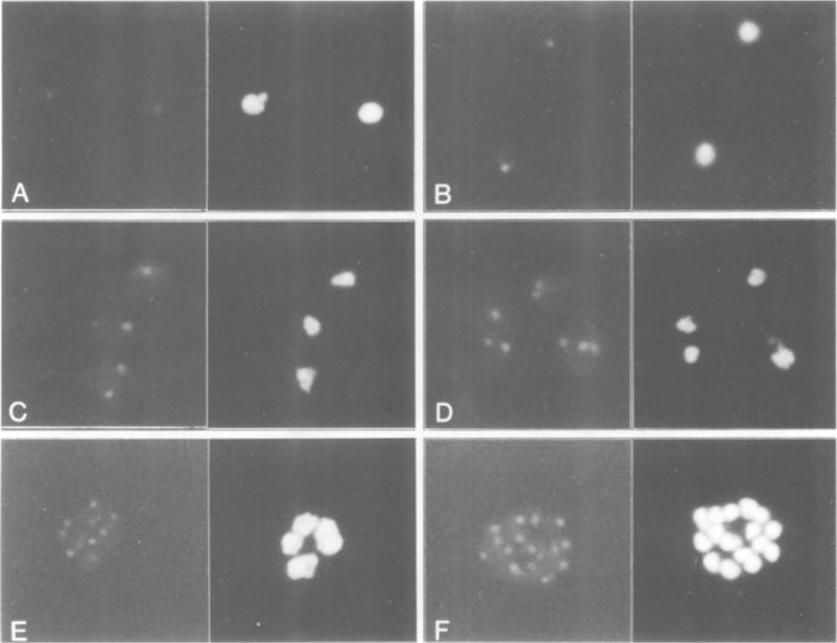
The identification of PfERD2 as a single discrete locus within the parasite. Thin blood smears were made from highly synchronized P.falciparum cultures, fixed and permeabilized, and incubated in affinity-purified PfERD2 antibody followed by incubation in FITC-conjugated goat anti-rabbit IgG (left panels). Nuclei were delineated by inclusion of 2 Ag/ml Hoechst in a final wash (right panels). Early ring stage infected erythrocytes, 10 h post-invasion (A); late ring stage infected erythrocytes, 22 h post-invasion (B); late trophozoite stage parasites, 34 h post-invasion (C and D); schizont stage parasite developing from four to eight nuclei (E); and late stage schizont, 16 nuclei (F). PfERD2 is tightly confined to a single focus of staining in the perinuclear region as seen by indirect immunofluorescence. This is redistributed by brefeldin A (BFA) to a diffuse pattern similar to that of parasite BiP, a marker for the ER; removal of the drug results in recovery of the single focus, consistent with the localization of PfERD2 to the parasite Golgi and its participation in a retrograde transport pathway to the ERElmendorf HG, Haldar K. Identification and localization of ERD2 in the malaria parasite Plasmodium falciparum: separation from sites of sphingomyelin synthesis and implications for organization of the Golgi. EMBO J. 1993 12(12):4763-73.
See original on MMP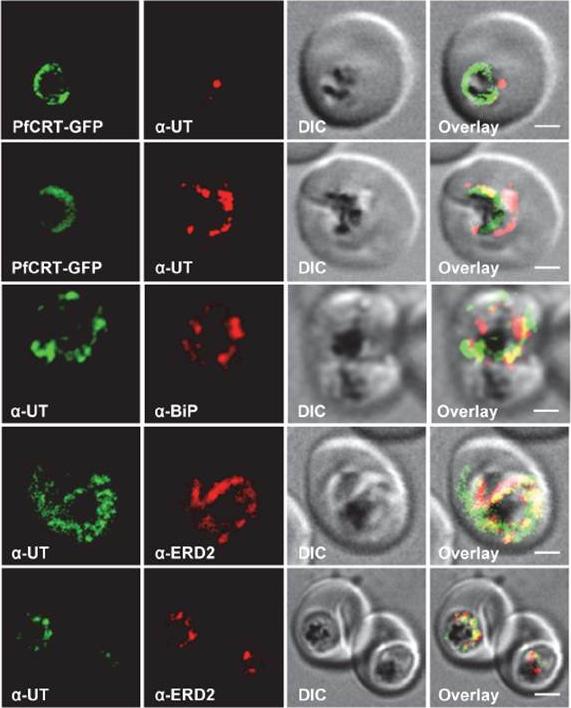
Subcellular localization of PfUT (ubiquitin-transferase). P. falciparum-infected erythrocytes at the trophozoite stage were fixed and analyzed by immunofluorescence assays using antisera to the ER marker BiP (rabbit, 1:1000), the Golgi marker ERD2 (rat, 1:500), and the N- (panels 1 and 5, rabbit, 1:3000; panel 3, mouse, 1:2000) and C-terminal domains of PfUT (panels 2 and 4, rabbit, 1:3000). Panel 1 shows a late ring stage parasite, the other panels show trophozoites. GFP fluorescence was detected, by confocal fluorescence microscopy, in parasites expressing episomally a PfCRT/GFP fusion protein. The different antisera raised against PfUT showed comparable results. Bar, 2 mm. Immunofluorescence microscopy partially co-localized PfUT with the ER marker BiP and the Golgi marker ERD2, but not with PfCRT.Sanchez CP, Liu CH, Mayer S, Nurhasanah A, Cyrklaff M, Mu J, Ferdig MT, Stein WD, Lanzer M. A HECT Ubiquitin-Protein Ligase as a Novel Candidate Gene for Altered Quinine and Quinidine Responses in Plasmodium falciparum. PLoS Genet. 2014 10(5):e1004382.
See original on MMP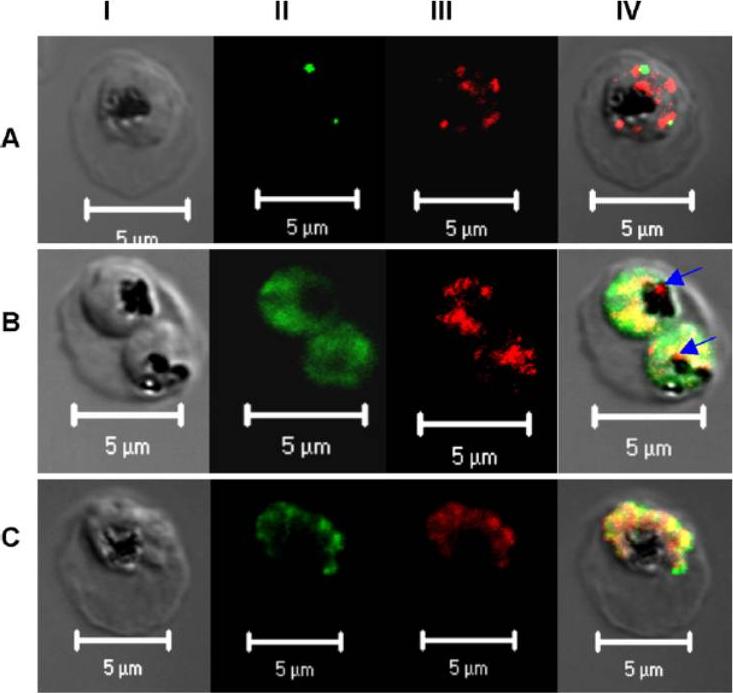
Subcellular localization of PfPRL. Micrographs represent the co-staining profiles of PfPRL with the Golgi marker PfErd2 (A), the ER marker PfBip (B), and the rhoptry/micronemal protein PfAMA-1 (C). Synchronous 3D7 parasite cultures were probed with purified rabbit anti-PfPRL in combination with antibodies to the compartmental markers. Binding of primary antibodies was detected using Alexa-Fluor 488 conjugated antibodies (organellar markers) and Alexa-Fluor 555-conjugated anti-rabbit IgG (PfPRL). Panel I is differential interference contrast image, panel II is fluorescence due to Alexa-Fluor 488 (green), panel III fluorescence due to Alexa-Fluor 555 (red), while panel IV is a merge of the first three panels. Yellow spots indicate overlap between PfPRL and the respective organellar marker, while blue arrows indicate food PfPRL-associated sites in the digestive vacuole.Pendyala PR, Ayong L, Eatrides J, Schreiber M, Pham C, Chakrabarti R, Fidock DA, Allen CM, Chakrabarti D. Characterization of a PRL protein tyrosine phosphatase from Plasmodium falciparum. Mol Biochem Parasitol. 2008 158(1):1-10.
See original on MMP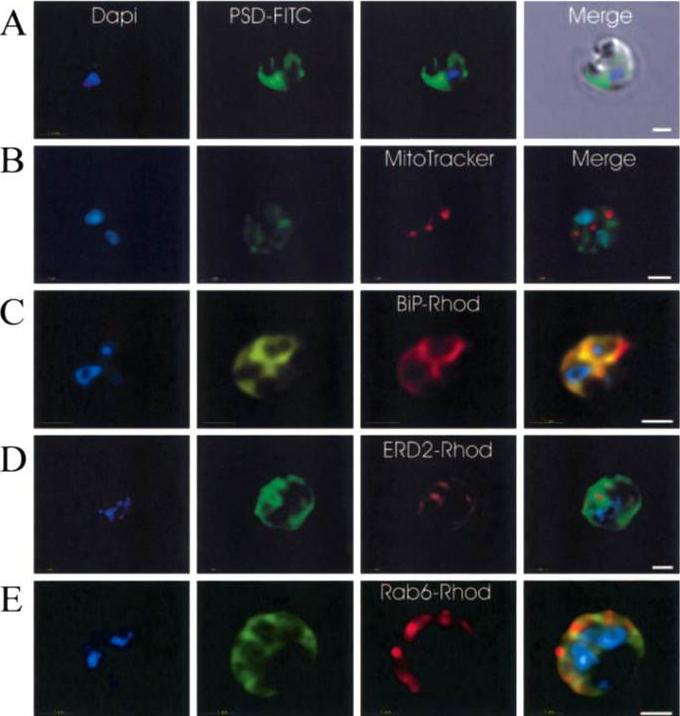
Immunofluorescence localization of PfPSD within P. falciparum-infected erythrocytes. A. FITC (which labels PfPSD) and DAPI (which labels nuclei) images were merged with the Nomarsky image to show the location of PSD labelling in the parasite. DAPI and FITC images were merged with the rhodamine channel corresponding to MitoTracker that labels the mitochondrion (B), BiP (PFI0875w)-rhodamine that labels the ER (C), ERD2 (PF13_0280)-rhodamine that labels the cis-Golgi (D) or Rab6 (PF11_0461)-rhodamine that labels the trans-Golgi (E). All the images except (A) correspond to one selected z-section image after digital deconvolution. The bar corresponds to 1 mm. The entire PfPSD labelling was clearly co-localized with the BiP endoplasmic reticulum marker.Baunaure F, Eldin P, Cathiard AM, Vial H. characterization of a non-mitochondrial type I phosphatidylserine decarboxylase in Plasmodium falciparum. Mol Microbiol. 2004 51:33-46. Copyright John Wiley & Sons Ltd. 2010.
See original on MMP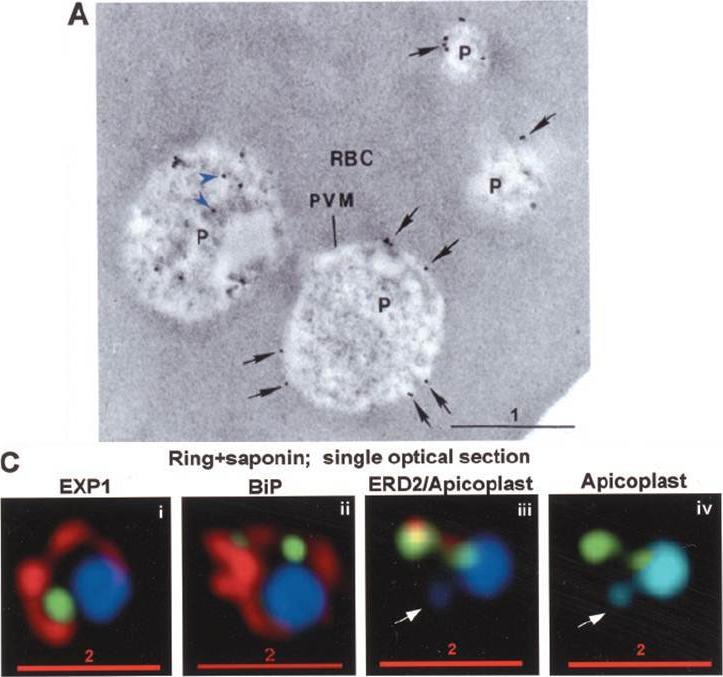
PTS-GFP is detected in the parasitophorous vacuole and in apposition with the Golgi in early rings. A, immunoelectron micrograph of rings showing localization of PTS-GFP in the parasitophorous vacuole and PVM (black arrows) as well as within the parasite (P; bluearrowheads). RBC, red blood cell. Scale bar, 1 mm. Gold particles detecting PTS-GFP are detected at the parasitophorous vacuole and vacuolar membrane (indicated by black arrows) of 6–12-h rings. Internal sites of PTS-GFP staining are also seen. PTS-GFP fluorescence showed some overlap with PfEXP1 a marker for the PVM as well as the Golgi marker PfERD2, consistent with the presence of label in the PV and internal secretory sites. C, i–iv: single optical sections showing green fluorescence in young rings permeabilized with 0.01% saponin relative to secretory markers PfEXP1, PfBiP, PfERD2 (shown in red in i–iii), and apicoplast DNA (marked with an arrow in iii and iv), as detected by indirect immunofluorescence and DeltaVision Microscopy In C, iv, the Hoechst stain is pseudo-colored cyan to facilitate visualization of apicoplast DNA. With 0.01% saponin (, i–iii) revealed loss of the peripheral green fluorescence (C, i–iii). Instead, the saponin-insensitive PTS-GFP associated green fluorescence was largely detectable in a single, major site within the parasite. As expected this site showed no overlap with the PVM marker PfEXP1 (C, i). It also showed no significant overlap with the ER marker BiP (C, ii). PTS-GFP showed no overlap with apicoplast DNA (C, iii and iv; apicoplast DNA is marked with an arrow. However, it was closely apposed to and partially overlapped with the PfERD2 Golgi site (C, iii).Cheresh P, Harrison T, Fujioka H, Haldar K. Targeting the malarial plastid via the parasitophorous vacuole. J Biol Chem. 2002 277(18):16265-77. PMID: 11815606
See original on MMP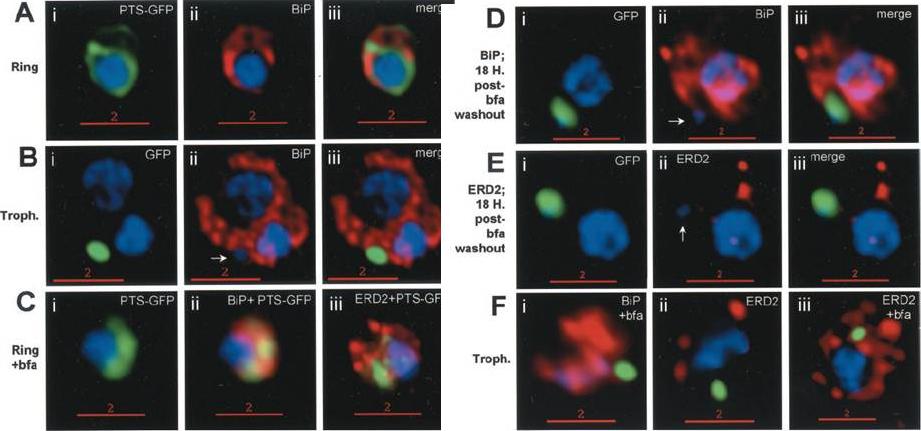
Stage-specific localization of apicoplast-targeted GFP and the effects of brefeldin A (bfa). Distribution of green fluorescence and PfBiP (red) in a early-ring (A, i–iii) and a trophozoite (B, i–iii; a late, ~33 h, trophozoite with twonuclei stained in blue is shown). Distribution of green fluorescence and indicated secretory marker (red) in: rings incubated with Bfa for 24 h (C, i–iii); rings incubated with Bfa for 24 h, washed, and grown for 18 h in absence of drug (D, i–iii; E, i–iii); and trophozoites incubated with or without Bfa (F, i–iii). Blue indicates DNA stained with Hoechst 33342. White arrows in B, ii, D, ii, and E, ii, indicate apicoplast DNA. Scale bars as indicated in microns. PTS-GFP in rings did not show significant overlap with the resident ER marker PfBiP. Nonetheless, its tubular distribution suggested that it resided in one or more membranous compartments. rings were allowed to mature in the presence of Bfa for 24 h (C, i–iii), the distribution of PTS-GFP as well as PfBiP (C, ii) were altered compared with either control rings or trophozoites shown in A and B. In the presence of Bfa, PfBiP lost its reticular staining and PTS-GFP accumulated in diffuse globular regions that show significant overlap with regions of PfBiP stain: however, PTS-GFP and BiP failed to show the identical distribution in Fig. 3C. The Golgi marker PfERD2 was also reorganized by Bfa treatment, consistent with the action of the drug on blocking transport through the Golgi (C, iii).Cheresh P, Harrison T, Fujioka H, Haldar K. Targeting the malarial plastid via the parasitophorous vacuole. J Biol Chem. 2002 277(18):16265-77.
See original on MMP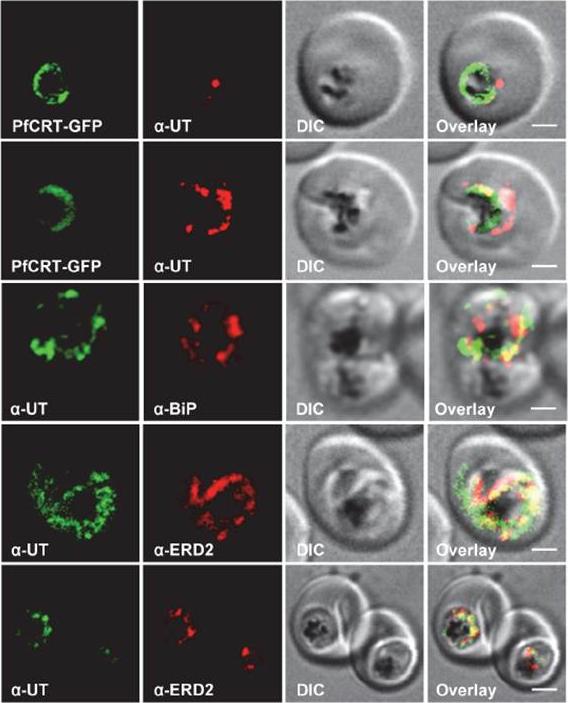
HECT ubiquitin-protein ligase (PfUT) localizes to the ER/Golgi complex. Subcellular localization of PfUT. P. falciparum-infected erythrocytes at the trophozoite stage were fixed and analyzed by immunofluorescence assays using antisera to the ER marker BiP, the Golgi marker ERD2, and the N- (panels 1 and 5, rabbit and C-terminal domains of PfUT. Panel 1 shows a late ring stage parasite, the other panels show trophozoites. GFP fluorescence was detected, by confocal fluorescence microscopy, in parasites expressing episomally a PfCRT/GFP fusion protein. The different antisera raised against PfUT showed comparable results. Bar, 2 mm. Immunofluorescence microscopy partially co-localized PfUT with the ER marker BiP and the Golgi marker ERD2, but not with PfCRT. Quantitative immunoelectron microscopy confirmed a predominant localization of PfUT at the ER/Golgi complex.Sanchez CP, Liu CH, Mayer S, Nurhasanah A, Cyrklaff M, Mu J, Ferdig MT, Stein WD, Lanzer M. A HECT ubiquitin-protein ligase as a novel candidate gene for altered quinine and quinidine responses in Plasmodium falciparum. PLoS Genet. 2014 10(5):e1004382.
See original on MMP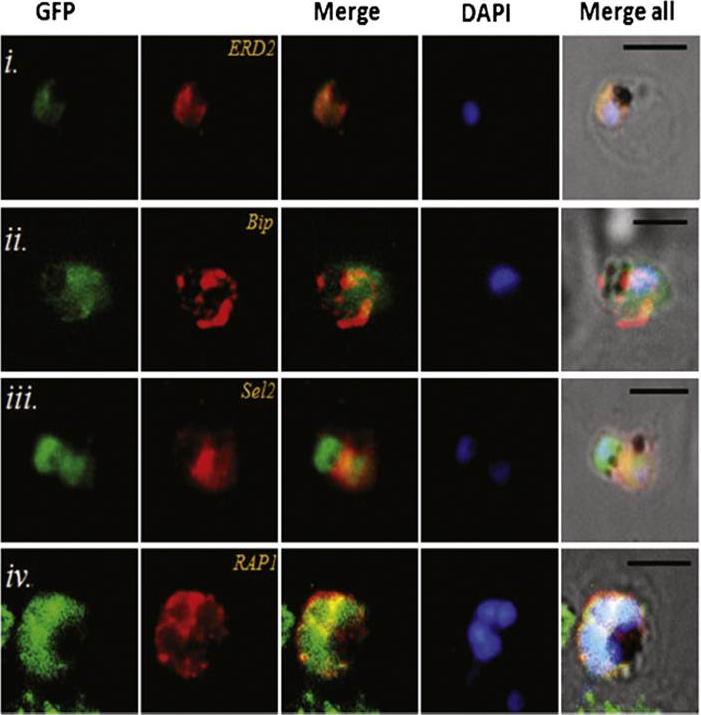
Transgenic parasites expressing Pfμ1–GFP were treated with Brefeldin A (BFA) and immunostained with antibodies specific to cis-Golgi apparatus marker ERD2 (i), endoplasmic reticulum marker, Bip (ii), cytoplasm localized, Sel2 (iii) and RAP1 (iv). The Pfμ1–GFP fusion protein colocalized with Sel2 as well as ERD2 in the parasite cytoplasm upon BFA treatment (i & iii). Parasite nuclei were stained with DAPI; scale bars denote 5 μm. Co-localization with antibodies to ERD2 (a cis-Golgi marker) and BiP, as well as the resident rhoptry protein RAP1 and the cytosolic protein Sel2 showed that Pfμ1 did not show a similar pattern as Bip or Sel2Pfμ1 showed a substantially similar staining pattern as ERD2 which is a Golgi marker (i–iii). Pfμ1 also did co-localize with RAP1 in BFA treated parasites (iv). These observations are consistent with Pfμ1 being Golgi associated at this life stage, consistent with its similar dynamics of redistribution upon brefeldin treatment.Kaderi Kibria KM, Rawat K, Klinger CM, Datta G, Panchal M, Singh S, Iyer GR, Kaur I, Sharma V, Dacks JB, Mohmmed A, Malhotra P. A role for adaptor protein complex 1 in protein targeting to rhoptry organelles in Plasmodium falciparum. Biochim Biophys Acta. 2015 1853(3):699-710.
See original on MMP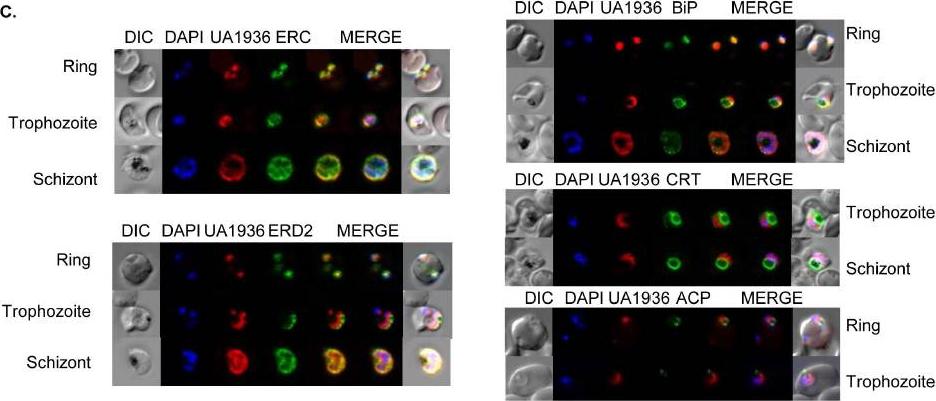
Fluorescence microscopy of UA1936(and inhibitor) and its localization in P. falciparum-infected red blood cells. P. falciparum-infected red blood cells (asynchronous cultures) were cultured with or without 100 mM UA1936 for 1 h and then fixed with 4% paraformaldehyde. In-cell click chemistry was performed with 2.5 mM Alexa 488 alkyne (green) or 2.5 TAMRA alkyne (red) for 30 min. For immunodetection, P. falciparum-infected red blood cells were incubated with specific antibodies against the endoplasmic reticulum markers ERC and BiP, against the cis-Golgi marker ERD2, against the food vacuole membrane marker CRT and against the apicoplast marker ACP.Penarete-Vargas DM, Boisson A, Urbach S, Chantelauze H, Peyrottes S, Fraisse L, Vial HJ. A chemical proteomics approach for the search of pharmacological targets of the antimalarial clinical candidate albitiazolium in Plasmodium falciparum using photocrosslinking and click chemistry. PLoS One. 2014 Dec 3;9(12):e113918. ·
See original on MMP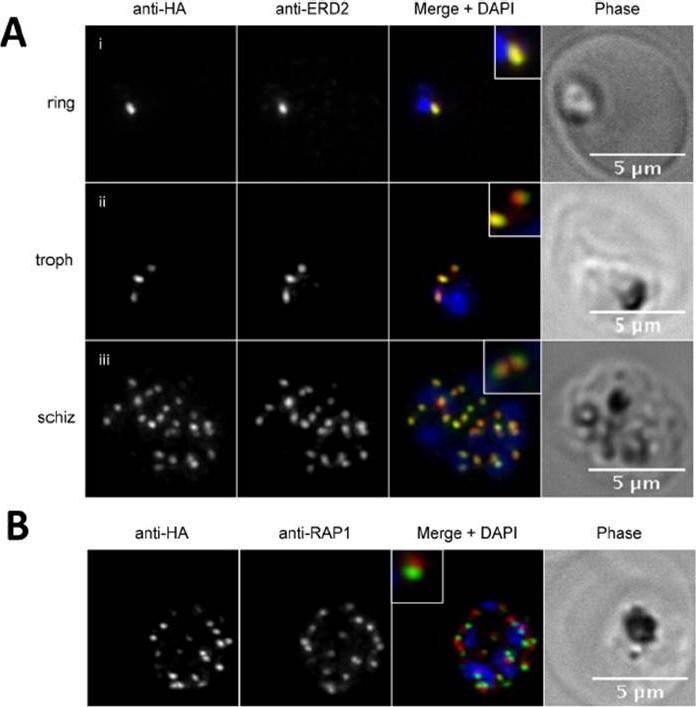
PfPRP2-RFA overlaps extensively with the Golgi apparatus throughout the erythrocytic cycle. (A) In ring stages, PfPRP2-RFA is found as a single punctate pattern colocalizing with the cis-Golgi marker ERD2 (Ai). In trophozoites, prior to nuclear division, the pattern associated with both PfPRP2-RFA and ERD2 becomes triple dots, showing the division of the Golgi, prior to nuclear replication (Aii). In schizont stages, themultiple PfPRP2-RFA signals still colocalize with the cis-Golgi protein ERD2 (Aiii). (B) In mature schizonts, PfPRP2-RFA is found in close proximity but does not overlap with the rhoptry marker RAP1. RAP1, rhoptry associated protein 1. Nuclei of parasites were stained with DAPI (blue). The fluorescence of PfPRP2-RFA is pseudocolored in green and other markers are in red. Scale bar represents 5μm.Hallée S, Richard D. Evidence that the Malaria Parasite Plasmodium falciparum Putative Rhoptry Protein 2 Localizes to the Golgi Apparatus throughout the Erythrocytic Cycle. PLoS One. 2015 Sep 16;10(9):e0138626.
See original on MMP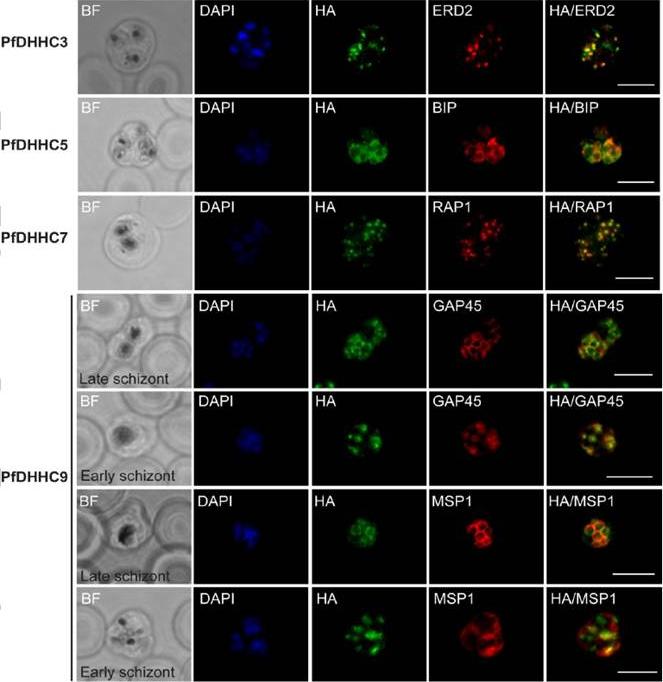
Expression and localisation of PfDHHC proteins in P. falciparum schizonts. Triple-HA-tagged PfDHHC proteins were localised by immunofluorescence using antibodies against the 3-HA tag (green). Immunofluorescence staining of each of the tagged PfDHHC proteins was compared against that of the following known localisation markers (red): ERD2 (Golgi marker), BIP (endoplasmic reticulum marker), RAP1 (rhoptry marker), GAP45 (inner membrane complex marker) and MSP1 (plasma membrane marker). Nuclear staining by DAPI is shown in blue. For the staining of PfDHHC9 with GAP45 and MSP1, both a late schizont, as well as an early schizont, is shown in order to differentiate between IMC and plasma membrane localisation. Scale bar: 5 μm.Tay CL, Jones ML, Hodson N, Theron M, Choudhary JS, Rayner JC. Study of Plasmodium falciparum DHHC palmitoyl-transferases identifies a role for PfDHHC9 in gametocytogenesis. Cell Microbiol. 2016 Apr 6.
See original on MMP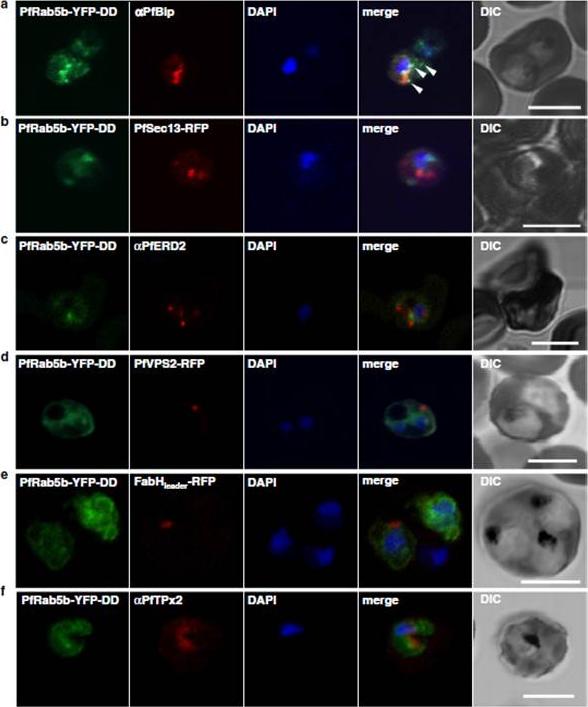
Localization of PfRab5b to a punctate compartment in the parasite cytoplasm. Triple staining with PfRab5b-YFP-DD (green), DAPI (blue) and one of the following markers (red): PfBip (a, ER), PfSec13-RFP (b, ER exit site), PfERD2 (c, Golgi), PfVPS2-RFP (d, putative multivesicular body/endosome), FabHleader-RFP (e, apicoplast), or PfTPx-2 (f, mitochondria) after 24 h incubation with Shld1. PfRab5b-YFP-DD localized adjacent to the Bip signal (arrowheads). Bars 5 μm.Ebine K, Hirai M, Sakaguchi M, Yahata K, Kaneko O, Saito-Nakano Y. Plasmodium Rab5b is secreted to the cytoplasmic face of the tubovesicular network in infected red blood cells together with N-acylated adenylate kinase 2. Malar J. 2016 15:323.
See original on MMP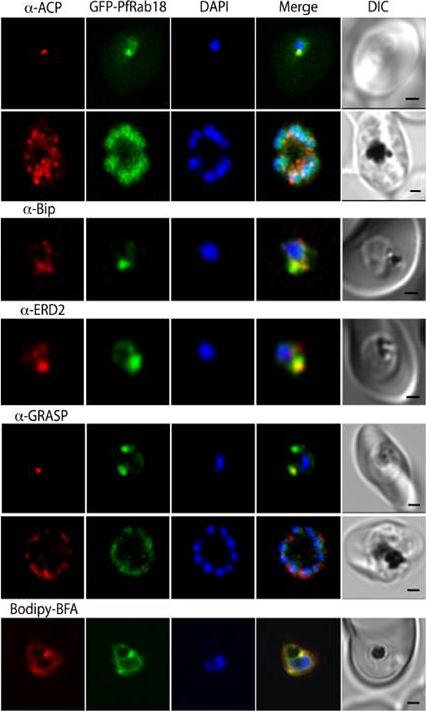
GFP-Rab18 fluorescence co-localizes with ER/Golgi, dense granules or microneme markers. GFP-PfRab1A fluorescence is distinct from the localization of markers for the apicoplast (ACP), the ER (Bip), the Golgi (ERD2 and GRASP), as well as from staining of the ER/Golgi with Bodipy BFA.Morse D, Webster W, Kalanon M, Langsley G, McFadden GI. Plasmodium falciparum Rab1A Localizes to Rhoptries in Schizonts. PLoS One. 2016 11(6):e0158174
See original on MMP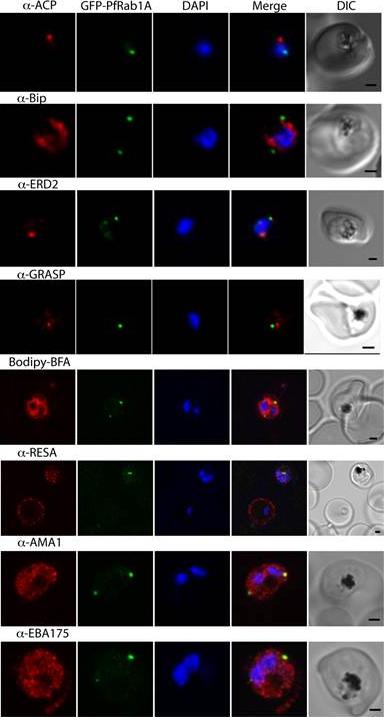
GFP-PfRab1A fluorescence does not co-localize with ER/Golgi, dense granules or microneme markers. GFP-PfRab1A fluorescence is distinct from the localization of markers for the apicoplast (ACP), the ER (Bip), the Golgi (ERD2 and GRASP), as well as from staining of the ER/Golgi with Bodipy BFA. GFP-Rab1A fluorescence is also distinct from the localization of markers for dense granules (RESA) or micronemes (AMA1 and EBA175).Morse D, Webster W, Kalanon M, Langsley G, McFadden GI. Plasmodium falciparum Rab1A Localizes to Rhoptries in Schizonts. PLoS One. 2016 Jun 27;11(6):e0158174
See original on MMP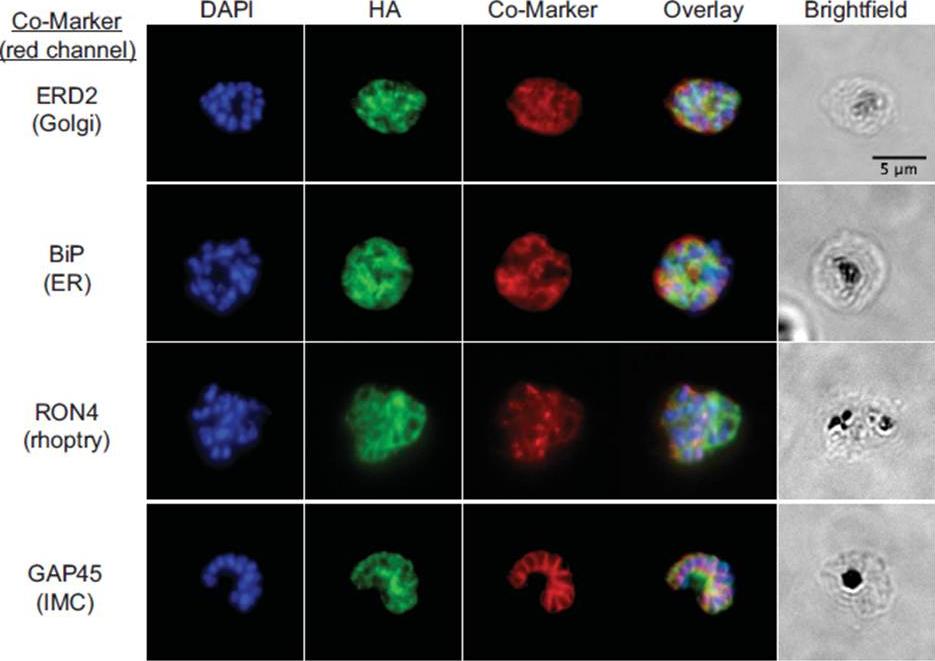
fixed schizont stage parasites were stained with rat α-HA antibody and co-stained with rabbit anti-PfERD2 (1:200), rabbit anti-PfBiP (1:500), mouse anti-PfRON4 (1:100), or rabbit anti-PfGAP45 (1:1000). Primary antibodies were detected with Alexa488-conjugated goat α-rat antibody (1:1000) and Alexa555-conjugated goat α-rabbit or goat anti-mouse (1:1000).Blomqvist K, DiPetrillo C, Streva VA, Pine S, Dvorin JD. Receptor for Activated C-Kinase 1 (PfRACK1) is required for Plasmodium falciparum intra-erythrocytic proliferation. Mol Biochem Parasitol. 2016 Oct 9. pii: S0166-6851(16)30129-3.
See original on MMP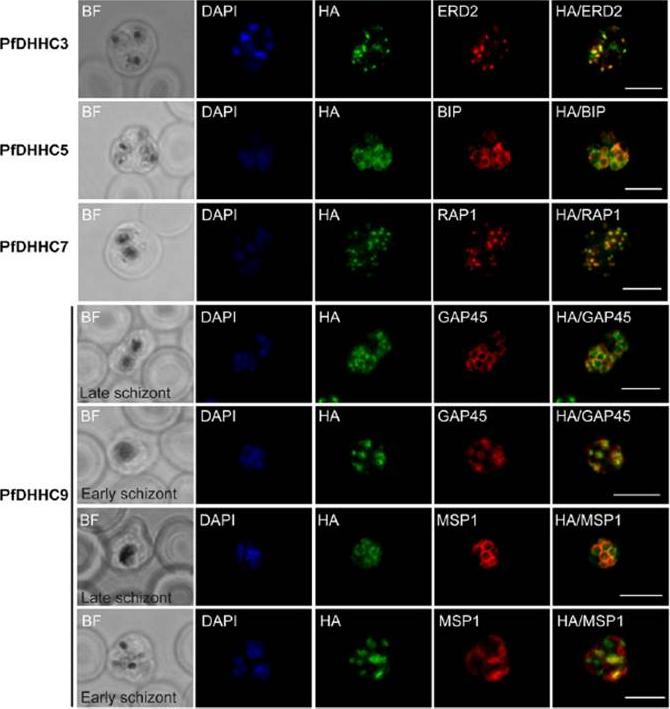
Expression and localization of PfDHHC proteins in Plasmodium falciparum schizonts. Triple-HA-tagged PfDHHC proteins were localized by immuno-fluorescence using antibodies against the 3-HA tag (green). Immunofluorescence staining of each of the tagged PfDHHC proteins was compared against that of the following known localization markers (red): ERD2 (Golgi marker), BIP (endoplasmic reticulum marker), RAP1 (rhoptry marker), GAP45 (inner membrane complex marker) and MSP1 (plasma membrane marker). Nuclear staining by DAPI is shown in blue. For the staining of PfDHHC9 with GAP45 and MSP1, a late schizont, as well as an early schizont, is shown in order to differentiate between inner membrane complex and plasma membrane localization. Scale bar: 5 μm.Tay CL, Jones ML, Hodson N, Theron M, Choudhary JS, Rayner JC. Study of Plasmodium falciparum DHHC palmitoyl transferases identifies a role for PfDHHC9 in gametocytogenesis. Cell Microbiol. 2016 18(11):1596-1610.
See original on MMPMore information
| PlasmoDB | PVX_114670 |
| GeneDB | PVX_114670 |
| Malaria Metabolic Pathways | Localisation images Pathways mapped to |
| Previous ID(s) | Pv114670 |
| Orthologs | PBANKA_1130200 , PCHAS_1129700 , PF3D7_1353600 , PKNH_1118100 , PVP01_1118100 , PY17X_1131600 |
| Google Scholar | Search for all mentions of this gene |