PVX_099320 glideosome-associated protein 50, putative (GAP50)
Disruptability [+]
| Species | Disruptability | Reference | Submitter | |
|---|---|---|---|---|
| P. berghei ANKA |
Refractory |
PlasmoGEM (Barseq) | PlasmoGEM | |
| P. falciparum 3D7 |
Possible |
USF piggyBac screen (Insert. mut.) | USF PiggyBac Screen | |
Mutant phenotypes [+]
None reported yet. Please press the '+' button above to add one.Imaging data (from Malaria Metabolic Pathways)
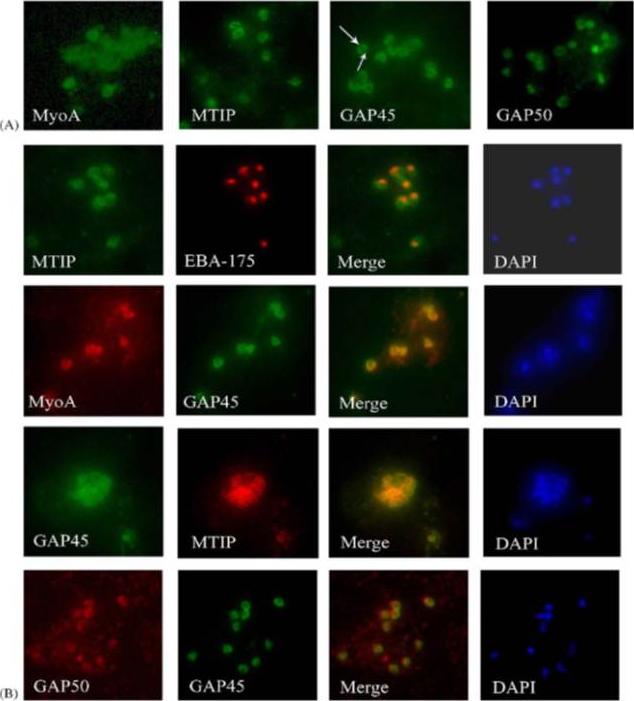
Components of the P. falciparum merozoite glideosome localize to the periphery of the parasite. (A) Indirect-immunofluorescence of individual glideosome components in late blood-stage parasites: Thin smears of P. falciparum infected erythrocytes were probed with anti-glideosome sera. The arrow indicates a gap in peripheral staining at the end of the merozoite, suggestive of an inner membrane complex (IMC) localization. (B) Indirect-immunofluorescence of late blood-stage parasites co-stained with antisera to other glideosome components and PfEBA-175: Glideosome antisera were used in co-immunofluorescence assays to assess co-localization. Anti-PfEBA-175 antisera recognize an invasion ligand that is localized to the micronemes, and is used as a marker for the apical end of the merozoite.Jones ML, Kitson EL, Rayner JC. Plasmodium falciparum erythrocyte invasion: a conserved myosin associated complex. Mol Biochem Parasitol. 2006 147:74-84. Copyright Elsevier 2009.
See original on MMP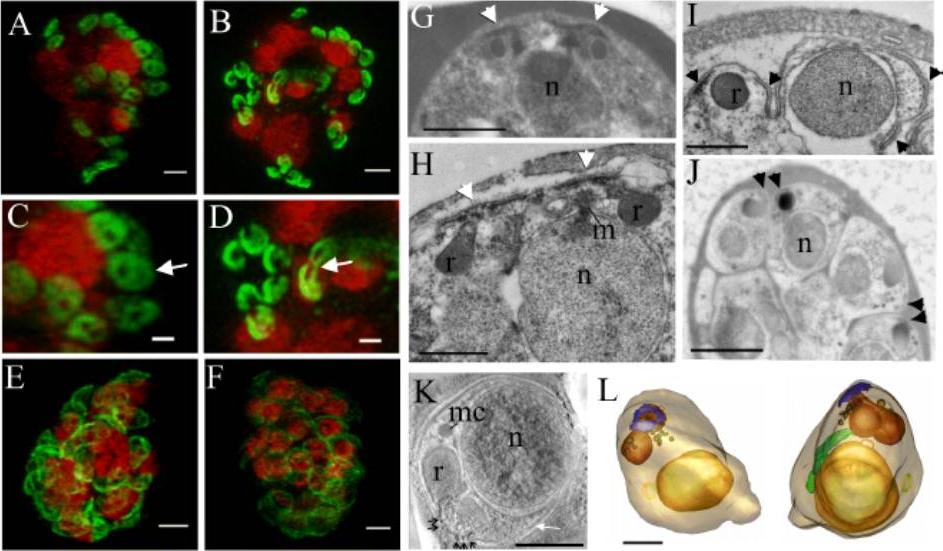
The IMC forms around the apical pore of developing merozoites. Structured illumination microscopy (3D-SIM) was performed on parasites expressing PfGAP50-GFP (green) labelled with DAPI nuclear stain (depicted in red). A,C. Schizont with eight nuclei, each with two associated PfGAP50-GFP-containing ellipsoids. Each ellipsoid has two unlabelled “pores” B,D. A schizont with eight nuclei that are undergoing division showing the PfGAP50-GFP-containing structures separating into claw-like structures. E,F. Mature schizonts in which the PfGAP50-GFP fluorescence is more evenly distributed around the periphery of the parasite. G,H. Transmission EM (TEM) images of early schizonts with electron-dense apical prominences with overlying membrane caps (arrowheads) forming in pairs on either side of a nucleus (n). The apical caps are connected to the underlying electron-dense rhoptries (r) and form close to a mitotic spindle (m). I. TEM image of a maturing schizont showing invagination of the parasite plasma membrane around the daughter cells and separation of the apical caps (arrowheads). J. TEM image of a mature schizont showing electron dense structures at the apical ends of closely apposed daughter cells (arrowheads). K. Selected virtual section (22 nm) through a tomogram showing a merozoite developing within a schizont. The electron dense polar rings are indicated with black arrows and the IMC with a white arrow. A microneme (mc) is visible. L,M. Rendered tomographic reconstructions of merozoites, generated from serial sections through the schizont shown in K, showing the nucleus in yellow, rhoptries in burnt orange, dense granules in brown, the polar rings in blue, the mitochondrion in green and a cytostomal ring as a yellow circle. Rotations of the 3D projection in A, B, E, and F are presented in Movies S1 – S4. Bars: A-F,G,J. = 1 μm, H,I,L,K = 500 nm.E, Maier AG, Klonis N, Maco B, Baum J, Turnbull L, Whitchurch CB, Dixonand MW, Tilley L. Tracking glideosome-associated protein-50 reveals the development and organization of the inner membrane complex of P. falciparum. Eukaryot Cell. 2011 10(4):556-64
See original on MMP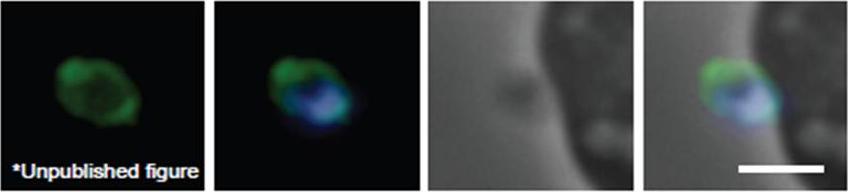
Schizont and merozoite culture was incubated overnight with 0.1µM cytochalasin D to inhibit invasion. Thin smeared on a glass slide, fixed in methanol, stained with rabbit anit-GAP50 polyclonal serum at 1:200 and secondary Alexa-Flor488 anti-Rabbit. Dual color fluorescence images were captured using a Carl Zeiss Axioskop 2 microscope at 100x using Oil immersion. Nucleus is marked with DAPI. Scale bar indicate 1µM. Invading merozoite is shown with GAP50 clearly marking the periphery (shown to be the IMC by immuno-EM).Baum J, Richard D, Healer J, Rug M, Krnajski Z, Gilberger TW, Green JL, Holder AA, Cowman AF. A conserved molecular motor drives cell invasion and gliding motility across malaria life cycle stages and other apicomplexan parasites. J Biol Chem. 2006 281(8):5197-208.
See original on MMP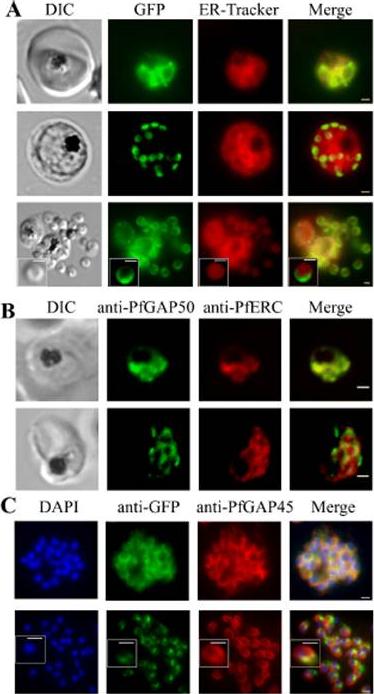
PfGAP50 is located in the ER prior to recruitment to the IMC. A. Parasites expressing PfGAP50-GFP (green) were incubated with ER-Tracker (depicted in red). The GFP and ER-Tracker fluorescence is co-located in trophozoite stage parasites. Upon relocation of GFP fluorescence to the apical ends of nascent merozoites during early schizont stage, the reticular ER-Tracker labelling persists. PfGAP50-GFP is concentrated at one pole of released merozoites while ER Tracker labels internal structures. B. Fixed cell immunofluorescence microscopy of the 3D7 parent strain labelled with antibodies raised against PfGAP50 (green) and PfERC (red). C. Immuno-fluorescence microscopy of PfGAP50-GFP transfectants at the mature and ruptured schizont stages labelled with anti-GFP (green), anti-PfGAP45 (red) and DAPI (blue). Bars = 1 μm. PfGAP50-GFP is located in the endoplasmic reticulum in early stage parasites, then redistributes to apical caps during the formation of daughter merozoites. In the final stage of schizogony the PfGAP50-GFP profile extends further around the merozoite surfaceYeoman JA, Hanssen E, Maier AG, Klonis N, Maco B, Baum J, Turnbull L, Whitchurch CB, Dixonand MW, Tilley L. Tracking glideosome-associated protein-50 reveals the development and organization of the inner membrane complex of P. falciparum. Eukaryot Cell. 2011 10(4):556-64.
See original on MMP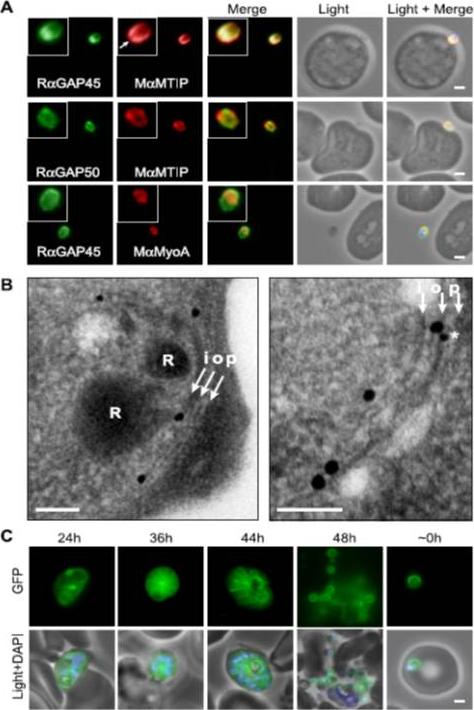
Localization of PfGAP proteins to the inner membrane complex. A, PfGAP50 and PfGAP45 co-localize with MTIP (a protein known to localize to the inner membrane complex) and partially with PfMyoA (known to associate with the innermembrane complex and merozoite apical tip (6, 38)). The white arrow in the MTIP inset points to an area of reduced fluorescence at the apical tip in a single z-stack, consistent with the absence of IMC at this position. B, immunoelectron microscopy confirms IMC localization for PfGAP45 (large 25-nm gold particle). The white arrows indicate trilaminar appearance of the IMC and merozoite plasma membrane. i, inner IMC membrane; o, outer IMC membrane; p, plasma membrane R, rhoptry. *, single anti-PfGAP50-conjugated gold particle found in close proximity to PfGAP45 but giving poor reactivity (typically 1 particle/merozoite). Scale bar in electron microscopy, 0.2 mm. C, PfGAP45-GFP shows fluorescence that concentrates to the merozoite periphery late in asexual development, consistent with the Scale bar in IFA, 1 mm.Baum J, Richard D, Healer J, Rug M, Krnajski Z, Gilberger TW, Green JL, Holder AA, Cowman AF. A conserved molecular motor drives cell invasion and gliding motility across malaria life cycle stages and other apicomplexan parasites. J Biol Chem. 2006 281(8):5197-208.
See original on MMP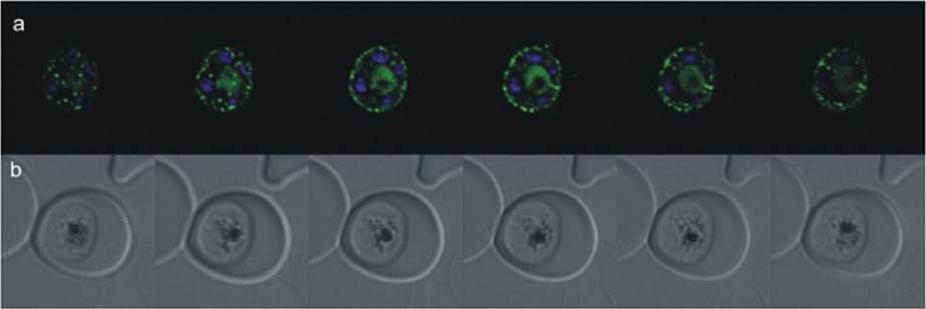
Confocal microscopy of an early schizont transgenically expressing SAP(1-367)-GFP (missing C-terminal sequence). GFP fluorescence was acquired by laser scanning through the parasite producing z stacks. a) Merged images of GFP and Hoechst staining of the nuclei; b) live images of the parasite taken in differential interference contrast. Capturing z-stack images through the parasite, it was possible to deduce the punctate GFP localization as being not only on the parasite’s surface where it might point to the formation of cytostomes but fluorescence was also seen in a more diffuse pattern in the cytosol, which could possibly represent a snapshot of vesicle trafficking. It is also localized in the food vacuole. This gene product was formerly described as anchoring glideosome associated protein 50 (GAP50).Müller IB, Knöckel J, Eschbach ML, Bergmann B, Walter RD, Wrenger C. Secretion of an acid phosphatase provides a possible mechanism to acquire host nutrients by Plasmodium falciparum. Cell Microbiol. 2010 12:677-91. Copyright John Wiley & Sons Ltd. 2010.
See original on MMP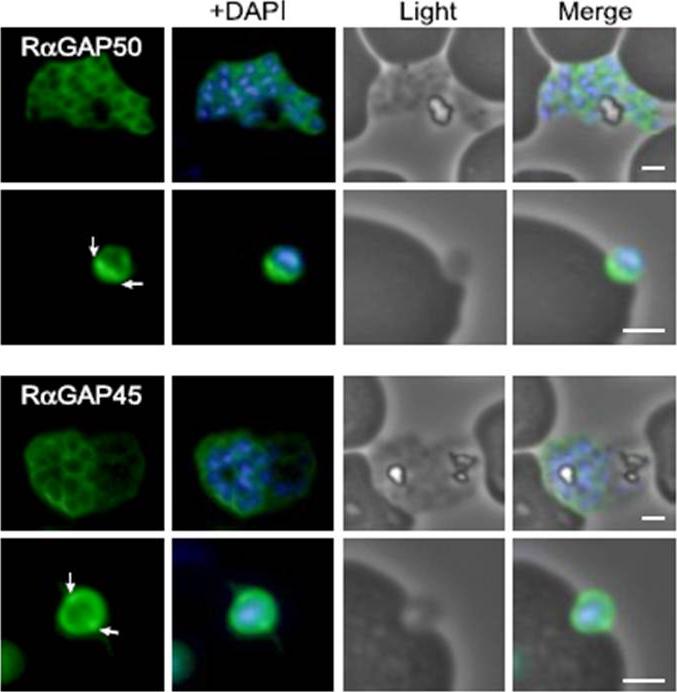
Characterization of PfGAP50 and PfGAP45 proteins. rabbit antiserum against recombinant PfGAP50 localizes to the merozoite periphery and remains following rupturing of schizont, consistent with localization to the IMC. The white arrows on putative invading merozoite point to interface with erythrocyte where increased fluorescence is detectable, possibly indicating concentration at the moving junction. Rabbit antiserum against recombinant PfGAP45 similarly suggests localization to the IMC. A second putative invading merozoite also suggests PfGAP45 is concentrated at the interface (white arrows) with the erythrocyte, consistent with the moving junction. Scale bar, 1 mm.Baum J, Richard D, Healer J, Rug M, Krnajski Z, Gilberger TW, Green JL, Holder AA, Cowman AF. A conserved molecular motor drives cell invasion and gliding motility across malaria life cycle stages and other apicomplexan parasites. J Biol Chem. 2006 281(8):5197-208.
See original on MMP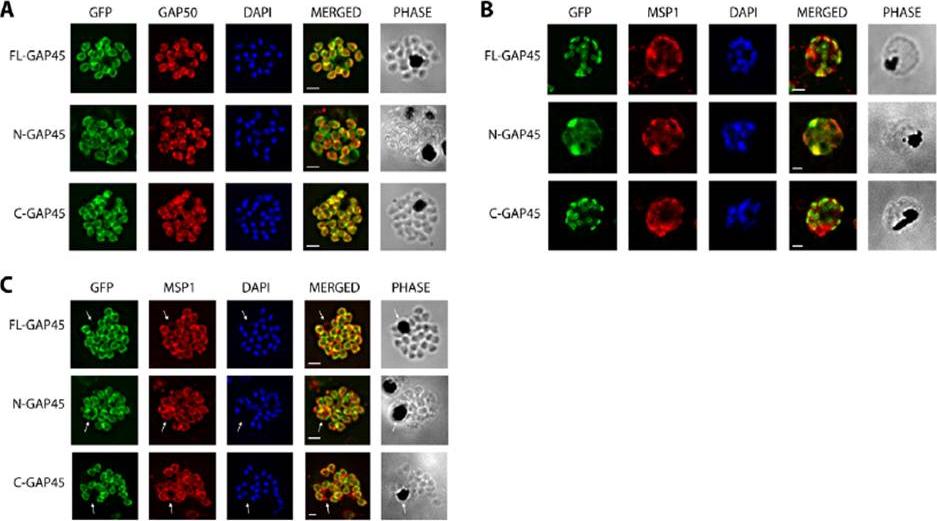
Immunofluorescent staining of GFP-tagged GAP45 variants with GAP50 in late stage schizonts. (A) Parasites were fixed and the location of GFP (green) and GAP50 (red), examined. FL-GAP45, C-GAP45 and N-GAP45 all appear to colocalise with GAP50 in segmented schizonts. (B) Staining with anti-MSP1 antibody (red) colocalises with only N-GFP in developing schizonts. FL-GAP45 AND C-GAP45 are present in distinctive ring-shaped structures at the periphery of the developing schizont. (C) In mature schizonts MSP1 (red) is present on membranes surrounding the residual body. N-GAP45 is also present on the residual body, but FL-GAP45 and C-GAP45 are absent. The white arrow in all images indicates the residual body. These data are consistent with a plasma membrane location for N-GAP45 and an inner membrane complex location for FL-GAP45. In all cases, parasite nuclei were stained with DAPI (blue); merged images are also shown. Scale bar is 2 mm.Immunofluorescent staining of GFP-tagged GAP45 variants with GAP50 in late stage schizonts. (A) Parasites were fixed and the location of GFP (green) and GAP50 (red), examined. FL-GAP45, C-GAP45 and N-GAP45 all appear to colocalise with GAP50 in segmented schizonts. (B) Staining with anti-MSP1 antibody (red) colocalises with only N-GFP in developing schizonts. FL-GAP45 AND C-GAP45 are present in distinctive ring-shaped structures at the periphery of the developing schizont. (C) In mature schizonts MSP1 (red) is present on membranes surrounding the residual body. N-GAP45 is also present on the residual body, but FL-GAP45 and C-GAP45 are absent. The white arrow in all images indicates the residual body. These data are consistent with a plasma membrane location for N-GAP45 and an inner membrane complex location for FL-GAP45. In all cases, parasite nuclei were stained with DAPI (blue); merged images are also shown. Scale bar is 2 mm.Ridzuan MA, Moon RW, Knuepfer E, Black S, Holder AA, Green JL. Subcellular Location, Phosphorylation and assembly into the motor complex of GAP45 during Plasmodium falciparum schizont development. PLoS One. 2012;7(3):e33845
See original on MMP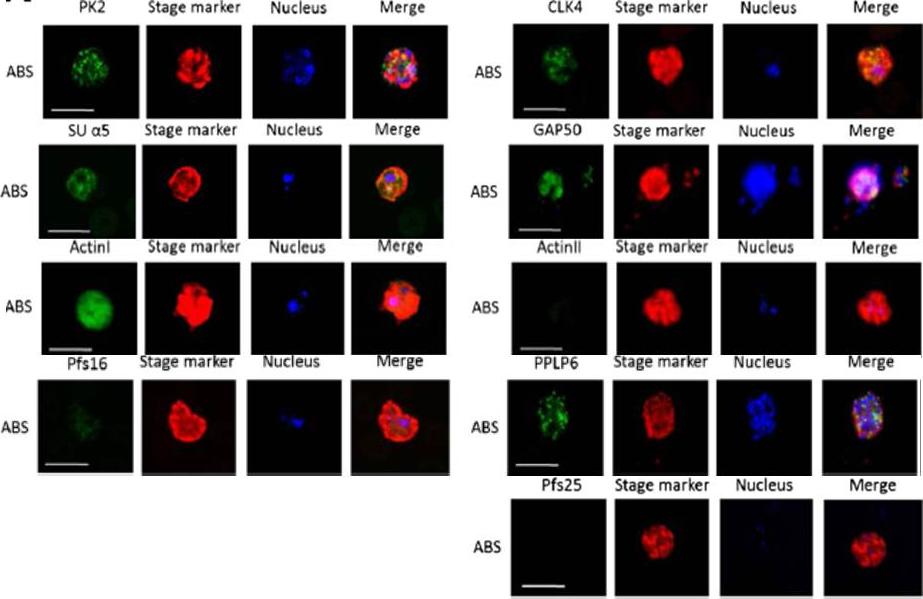
Immunofluorescence assays, using specific antibodies, detected the proteins of interest (in green) in asexual blood stage (ABS) parasites, using MSP1 (in red). Nuclei were highlighted by Hoechst nuclear stain (in blue). Bar, 5 μm. Ngwa CJ, Scheuermayer M, Mair GR, Kern S, Brügl T, Wirth CC, Aminake MN, Wiesner J, Fischer R, Vilcinskas A, Pradel G. Changes in the transcriptome of the malaria parasite Plasmodium falciparum during the initial phase of transmission from the human to the mosquito. BMC Genomics. 2013 Apr 15;14:256
See original on MMP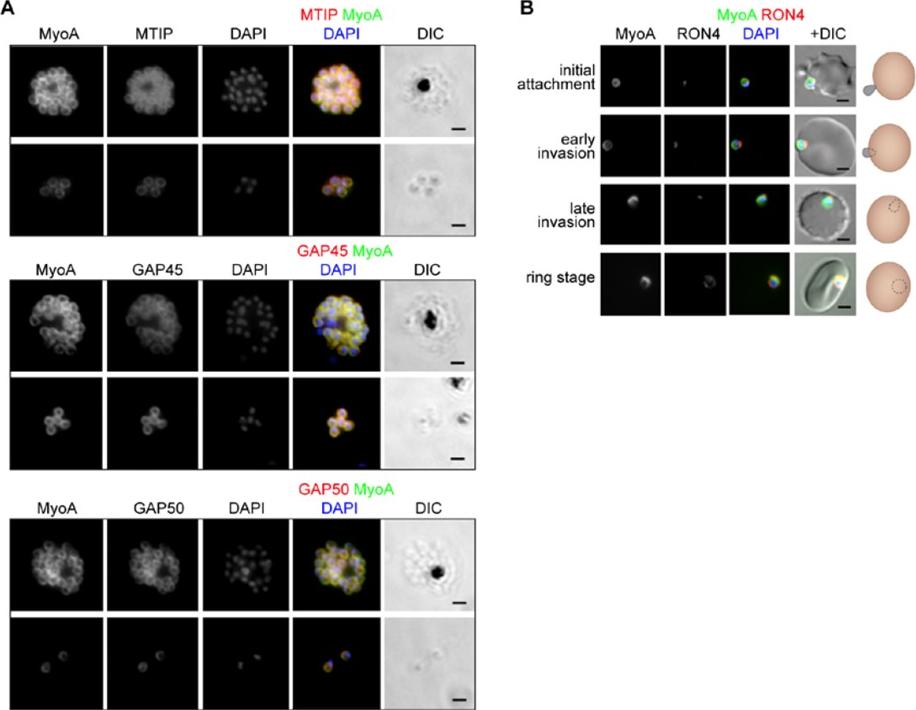
The location of MyoA during P. falciparum merozoite invasion. (A) MyoA-GFP is located at the periphery of developing intracellular (top row of each pair of images) and free extracellular merozoites (bottom row), and is colocalized with antibodies specific for IMC proteins MTIP, GAP45 and GAP50. In the merged colour image the MyoA-GFP signal is green and antibodies specific for the IMC proteins are red; nuclei are stained blue with DAPI. The DIC image is also shown. (B) Individual merozoites are captured at different stages of invasion from initial attachment, through early and late invasion to the intracellular ring stage. MyoA-GFP remains peripheral whereas RON4, initially in the apical rhoptry neck, relocates during invasion. Merged colour images with MyoA-GFP (green), RON4 (red), and nuclei (blue) and DIC images are also shown, together with a schematic of each cell-pair. Scale bar: 2 μm.Green JL, Wall RJ, Vahokoski J, Yusuf NA, Ridzuan MAM, Stanway RR, Stock J, Knuepfer E, Brady D, Martin SR, Howell SA, Pires IP, Moon RW, Molloy JE, Kursula I, Tewari R, Holder AA. Compositional and expression analyses of the glideosome during the Plasmodium life cycle reveal an additional myosin light chain required for maximum motility. J Biol Chem. 2017 Sep 11.
See original on MMPMore information
| PlasmoDB | PVX_099320 |
| GeneDB | PVX_099320 |
| Malaria Metabolic Pathways | Localisation images Pathways mapped to |
| Previous ID(s) | Pv099320 |
| Orthologs | PBANKA_0819000 , PCHAS_0819300 , PF3D7_0918000 , PKNH_0716000 , PVP01_0716400 , PY17X_0822300 |
| Google Scholar | Search for all mentions of this gene |