PVP01_1440900 glideosome-associated protein 45, putative (GAP45)
Disruptability [+]
| Species | Disruptability | Reference | Submitter | |
|---|---|---|---|---|
| P. falciparum 3D7 |
Possible |
USF piggyBac screen (Insert. mut.) | USF PiggyBac Screen | |
Mutant phenotypes [+]
None reported yet. Please press the '+' button above to add one.Imaging data (from Malaria Metabolic Pathways)
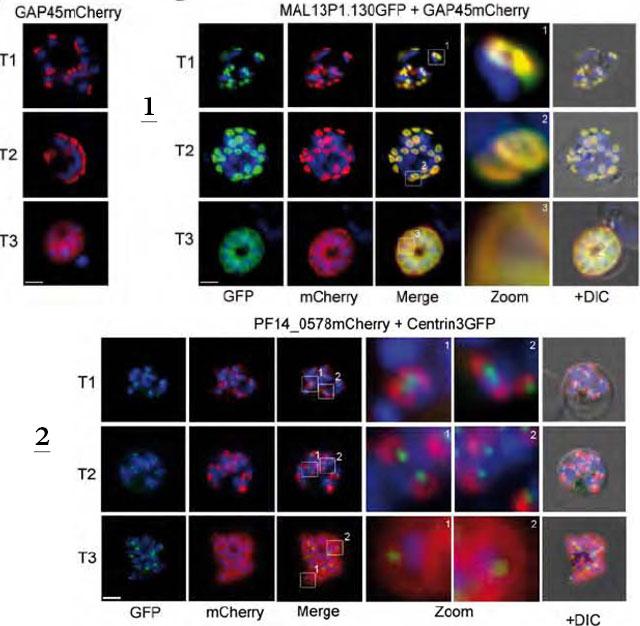
1. Co-localization of the TMD protein MAL13P1.130 and the glideosome associated protein GAP45. GAP45 was episomally expressed as mCherry fusion protein and localized in different schizont stages (left). Double transgenic cell line expressing MAL13P1.130-GFP and GAP45-mCherry reveals identical spatial distribution (right).2. Coexpression of the centrosome marker PfCentrin3-GFP and PF14_0578-mCherry shows a very close association between the forming IMC and the centrosome, as PfCentrin3 marks the centre of the developing IMC cramp-structure. Nuclei stained with DAPI. Scale bars, 2 μm.Kono M, Herrmann S, Loughran NB, Cabrera A, Engelberg K, Lehmann C, Sinha D, Prinz B, Ruch U, Heussler V, Spielman T, Parkinson J, Gilberger TW. Evolution and Architecture of the Inner Membrane Complex in Asexual and Sexual Stages of the Malaria Parasite. Mol Biol Evol. 2012 29(9):2113-32
See original on MMP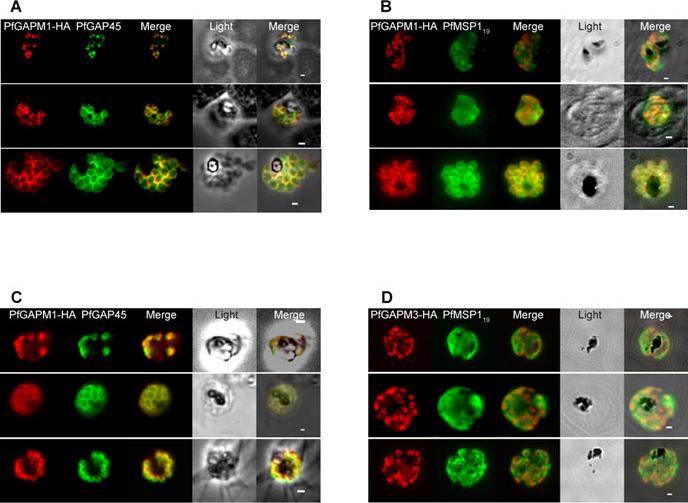
In Plasmodium falciparum the HA-tagged GAPMs (Glideosome Associated Protein with Multiple membrane spans) co-localise with the inner membrane complex protein GAP45 but not the plasma membrane protein MSP119. Immunofluorescence images of PfGAPM1-HA (A & B) and PfGAPM3-HA (C & D) expressing P. falciparum schizonts probed with an anti-HA antibody and antibodies to either the IMC-resident protein PfGAP45 (A & C) or the MSP119 subunit of the plasma membrane protein MSP1 PFI1475w (B & D). PfGAPM1-HA and PfGAPM3-HA co-localize with PfGAP45 PFL1090w throughout development but not with MSP119 particularly during early schizogony.Bullen HE, Tonkin CJ, O'Donnell RA, Tham WH, Papenfuss AT, Gould S, Cowman AF, Crabb BS, Gilson PR. A novel family of apicomplexan glideosome associated proteins with an inner-membrane anchoring role. J Biol Chem. 2009 284:25353-63.
See original on MMP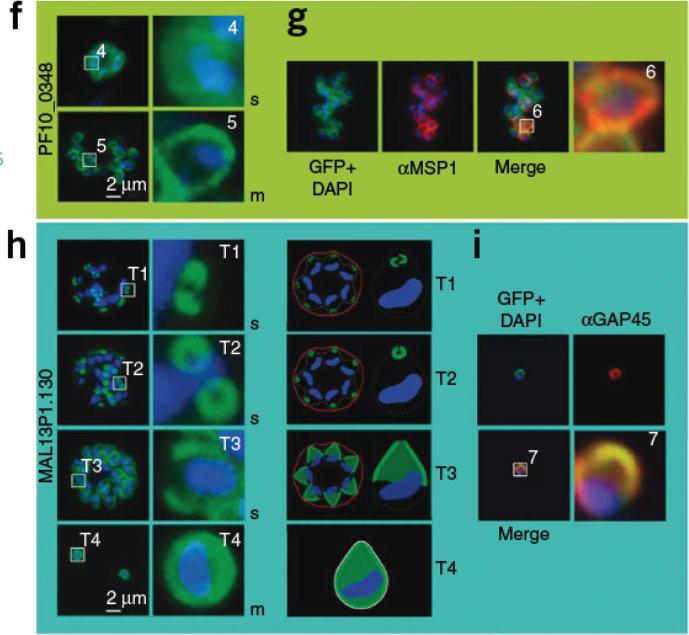
Boxed regions are numbered and depicted in higher magnification to the right. The nucleus is stained with DAPI (blue). f) PF10_0348-GFP (green) localized to the surface of schizonts and free merozoites in unfixed parasites. g) PF10_0348-GFP co-localized with the surface protein MSP-1 (red) in fixed parasites. Dynamics of MAL13P1.130-GFP (green) during schizogony in unfixed parasites. In early schizogony (T1), MAL13P1.130-GFP emerged as a cramp-like-structure at the apical tip of forming merozoites (h). This structure develops to be ring-like (T2) before becoming evenly distributed at the periphery of the nascent merozoite (T3-4). The third row shows a schematic representation. For confocal three-dimensional reconstitution,Hu G, Cabrera A, Kono M, Mok S, Chaal BK, Haase S, Engelberg K, Cheemadan S, Spielmann T, Preiser PR, Gilberger TW, Bozdech Z. Transcriptional profiling of growth perturbations of the human malaria parasite Plasmodium falciparum. Nature Biotechnol. 2010 28:91-8.
See original on MMP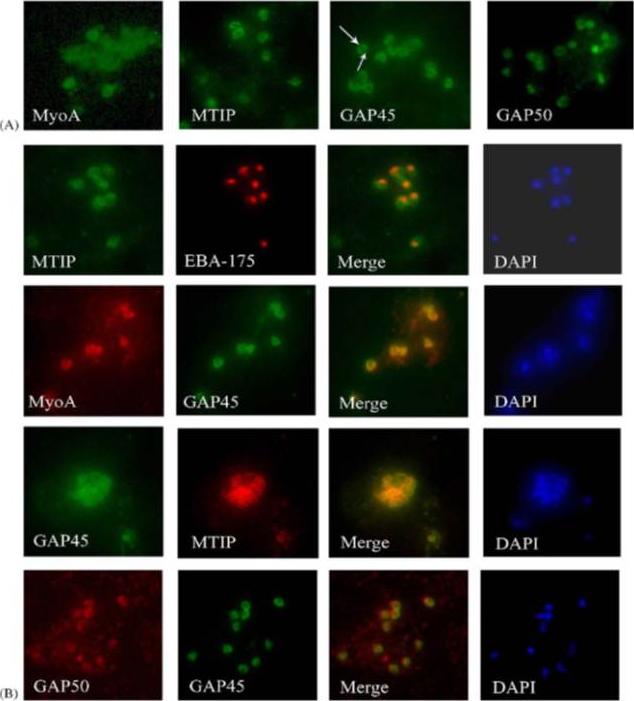
Components of the P. falciparum merozoite glideosome localize to the periphery of the parasite. (A) Indirect-immunofluorescence of individual glideosome components in late blood-stage parasites: Thin smears of P. falciparum infected erythrocytes were probed with anti-glideosome sera. The arrow indicates a gap in peripheral staining at the end of the merozoite, suggestive of an inner membrane complex (IMC) localization. (B) Indirect-immunofluorescence of late blood-stage parasites co-stained with antisera to other glideosome components and PfEBA-175: Glideosome antisera were used in co-immunofluorescence assays to assess co-localization. Anti-PfEBA-175 antisera recognize an invasion ligand that is localized to the micronemes, and is used as a marker for the apical end of the merozoite.Jones ML, Kitson EL, Rayner JC. Plasmodium falciparum erythrocyte invasion: a conserved myosin associated complex. Mol Biochem Parasitol. 2006 147:74-84. Copyright Elsevier 2009.
See original on MMP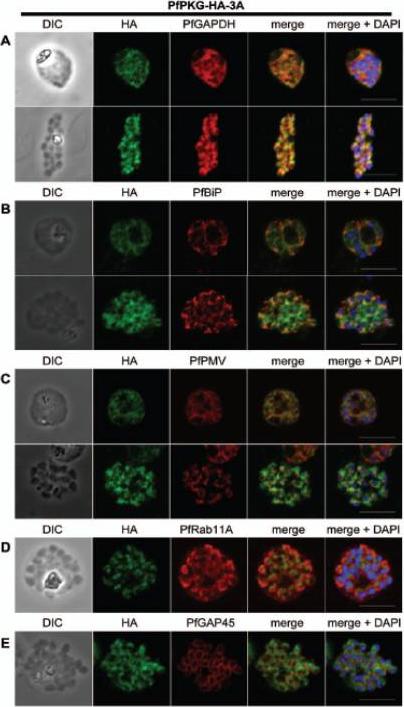
Subcellular location of PfPKG in mature schizonts. Dual immunofluorescent detection of PfPKG-HA in fixed smears of early and late schizonts of the PfPKG-HA-3A clone together with (A) GAPDH; (B) BiP; (C) PMV; (D) Rab11A and (E) GAP45. Representative images are shown for each antibody, together with bright field images (first column) and parasite nuclei stained with DAPI (in the merged image). Bars ,5 mm. Significant overlap between the PKG-HA and GAPDH in early and late blood stage schizonts (A), both are in the cytosol. PfPKG-HA also partially overlapped with that of the ER membrane protein PMV and that of the ER lumen marker PfBiP (B and C) in both early and late schizonts. Rab11A is thought to be involved in vesicle trafficking and localises to the rhoptries and the inner membrane complex (IMC) at the apical end of the merozoite. In mature schizonts, the location of PKG-HA appeared to be largely distinct from that of Rab11A (D). PKG-HA appeared to be absent from the IMC in segmented schizonts, since it did not colocalise with the IMC marker, the glideosome associated protein 45. Hopp CS, Flueck C, Solyakov L, Tobin A, Baker DA. Spatiotemporal and Functional Characterisation of the Plasmodium falciparum cGMP-Dependent Protein Kinase. PLoS One. 2012;7(11):e48206.
See original on MMP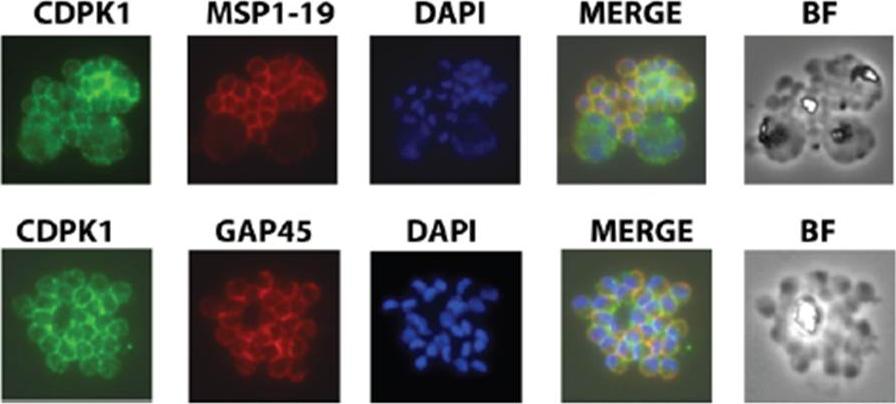
Expression of functional CDPK1 fusion proteins in 3D7 P. falciparum parasites Immunolocalisation of CDPK1 in late schizont stage parasites showing co-localisation with MSP1-19 and GAP45, proteins which reside on the plasma membrane and inner membrane complex, respectively. CDPK1 was detected with CDPK1-specific mouse monoclonal 3E6 and the other proteins with polyclonal rabbit antibodies .Azevedo MF, Sanders PR, Krejany E, Nie CQ, Fu P, Bach LA, Wunderlich G, Crabb BS, Gilson PR. Inhibition of Plasmodium falciparum CDPK1 by conditional expression of its J-domain demonstrates a key role in schizont development. Biochem J. 2013 452(3):433-41
See original on MMP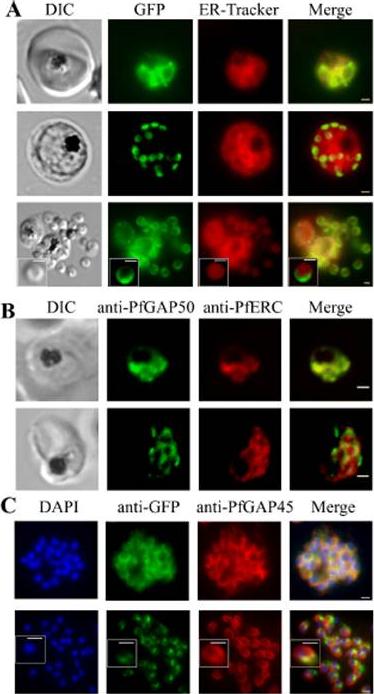
PfGAP50 is located in the ER prior to recruitment to the IMC. A. Parasites expressing PfGAP50-GFP (green) were incubated with ER-Tracker (depicted in red). The GFP and ER-Tracker fluorescence is co-located in trophozoite stage parasites. Upon relocation of GFP fluorescence to the apical ends of nascent merozoites during early schizont stage, the reticular ER-Tracker labelling persists. PfGAP50-GFP is concentrated at one pole of released merozoites while ER Tracker labels internal structures. B. Fixed cell immunofluorescence microscopy of the 3D7 parent strain labelled with antibodies raised against PfGAP50 (green) and PfERC (red). C. Immuno-fluorescence microscopy of PfGAP50-GFP transfectants at the mature and ruptured schizont stages labelled with anti-GFP (green), anti-PfGAP45 (red) and DAPI (blue). Bars = 1 μm. PfGAP50-GFP is located in the endoplasmic reticulum in early stage parasites, then redistributes to apical caps during the formation of daughter merozoites. In the final stage of schizogony the PfGAP50-GFP profile extends further around the merozoite surfaceYeoman JA, Hanssen E, Maier AG, Klonis N, Maco B, Baum J, Turnbull L, Whitchurch CB, Dixonand MW, Tilley L. Tracking glideosome-associated protein-50 reveals the development and organization of the inner membrane complex of P. falciparum. Eukaryot Cell. 2011 10(4):556-64.
See original on MMP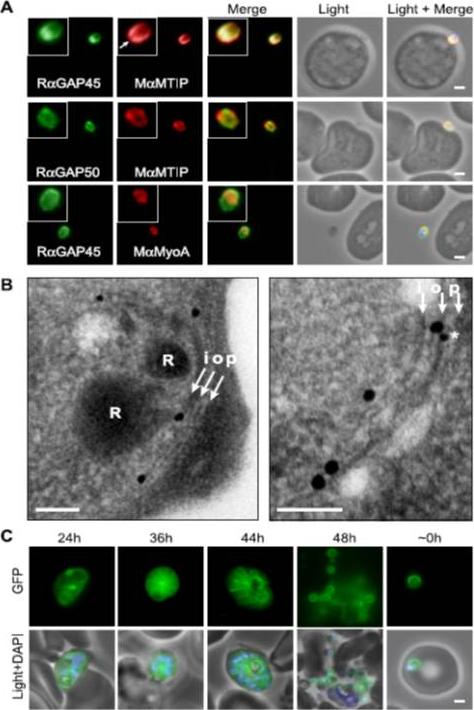
Localization of PfGAP proteins to the inner membrane complex. A, PfGAP50 and PfGAP45 co-localize with MTIP (a protein known to localize to the inner membrane complex) and partially with PfMyoA (known to associate with the innermembrane complex and merozoite apical tip (6, 38)). The white arrow in the MTIP inset points to an area of reduced fluorescence at the apical tip in a single z-stack, consistent with the absence of IMC at this position. B, immunoelectron microscopy confirms IMC localization for PfGAP45 (large 25-nm gold particle). The white arrows indicate trilaminar appearance of the IMC and merozoite plasma membrane. i, inner IMC membrane; o, outer IMC membrane; p, plasma membrane R, rhoptry. *, single anti-PfGAP50-conjugated gold particle found in close proximity to PfGAP45 but giving poor reactivity (typically 1 particle/merozoite). Scale bar in electron microscopy, 0.2 mm. C, PfGAP45-GFP shows fluorescence that concentrates to the merozoite periphery late in asexual development, consistent with the Scale bar in IFA, 1 mm.Baum J, Richard D, Healer J, Rug M, Krnajski Z, Gilberger TW, Green JL, Holder AA, Cowman AF. A conserved molecular motor drives cell invasion and gliding motility across malaria life cycle stages and other apicomplexan parasites. J Biol Chem. 2006 281(8):5197-208.
See original on MMP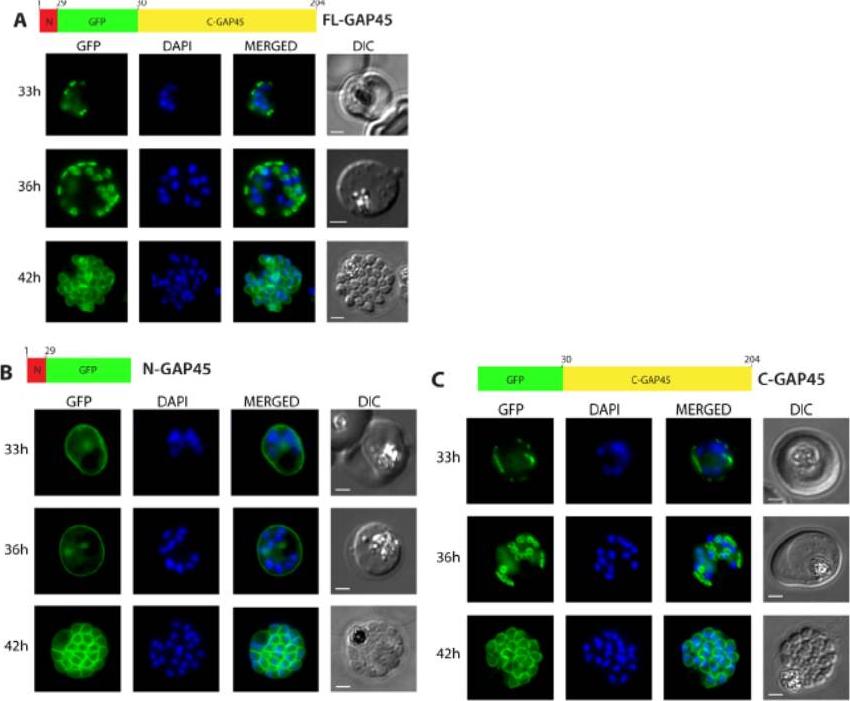
Subcellular localisation of GFP-tagged GAP45 protein and truncated derivatives. The location of (A) GFP-tagged wild type GAP45 (FL-GAP45), (B) GFP-tagged C- terminally truncated GAP45 (NGAP45), and (C) GFP-tagged N-terminally truncated proteins (C-GAP45) during schizont development. Transfected parasite populations were synchronized and examined at 33, 36 and 42 h post invasion by fluorescence microscopy to detect GFP (green). Nuclei were labelled with Hoechst stain (blue) prior to analysis. The merged image of the two is also shown together with the corresponding differential interference contrast (DIC) picture. Scale bar is 2 mm. In the early schizont stages small, localised structures of GFP signal were observed around the parasite’s periphery in both FL-GAP45 and C-GAP45, typical of an IMC location. N-GAP45 protein produced a different pattern of fluorescence probably corresponding to the parasite plasma membrane. At the late schizont stage (42 h post invasion) the GFP signal was detected at the periphery of merozoites developing within the schizont for all GAP45 variants, and at this time point the putative IMC and parasite plasma membrane patterns were indistinguishable.Ridzuan MA, Moon RW, Knuepfer E, Black S, Holder AA, Green JL. Subcellular Location, Phosphorylation and assembly into the motor complex of GAP45 during Plasmodium falciparum schizont development. PLoS One. 2012;7(3):e33845
See original on MMP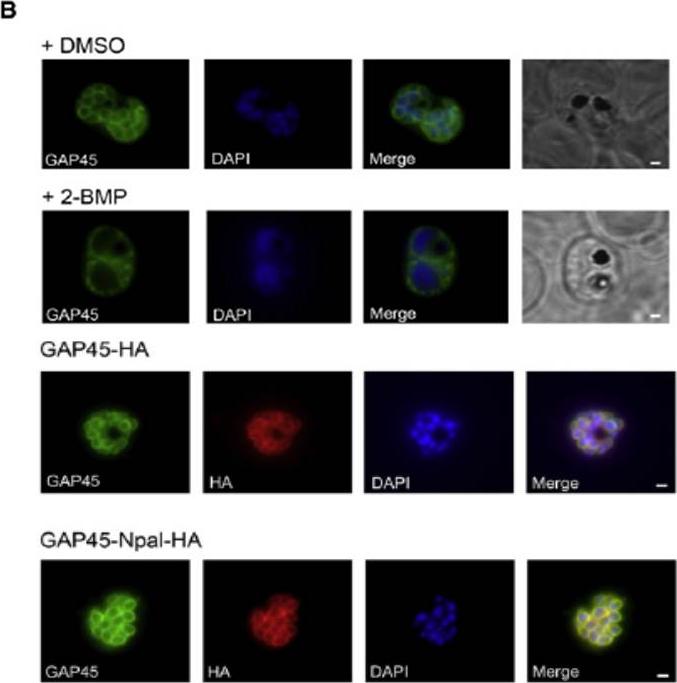
(B) Effect of 2-BMP (2-bromohexadecanoic acid) on PfGAP45 localization. Late schizonts (44–48 hr after invasion) were treated with 50 mM 2-BMP, and PfGAP45 targeting to the inner membrane complex (IMC) was assessed with anti-PfGAP45 antibodies in immunofluorescence assays. DMSO treated parasites were used as a control. Scale bars represent 1 mm. (D) Mutation of the PfGAP45 N-terminal palmitoylation site does not prevent IMC targeting. Anti-HA and anti-PfGAP45 antisera were used to follow localization of tagged and endogenous GAP45 by immunofluorescence in late schizont stage PfGAP45-HA and PfGAP45-Npal-HA expressing parasites. Scale bars represent 1 mm.Jones ML, Collins MO, Goulding D, Choudhary JS, Rayner JC. Analysis of protein palmitoylation reveals a pervasive role in Plasmodium development and pathogenesis. Cell Host Microbe. 2012 12(2):246-58.
See original on MMP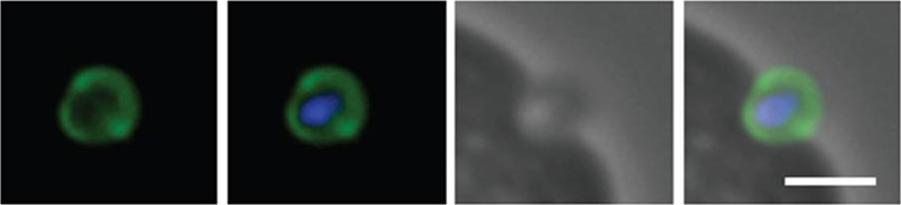
Schizont and merozoite culture was incubated overnight with 0.1µM cytochalasin D to inhibit invasion. Thin smeared on a glass slide, fixed in methanol, stained with rabbit anit-GAP45 polyclonal serum at 1:200 and secondary Alexa-Flor488 anti-Rabbit. Dual colour fluorescence images were captured using a Carl Zeiss Axioskop 2 microscope at 100x using Oil imersion. Nucleus is marked with DAPI. Scale bar indicate 1µM. Invading merozoite is shown with GAP45 clearly marking the periphery (shown to be the IMC by immuno-EM) and suggesting of localization to tight junction.Baum J, Richard D, Healer J, Rug M, Krnajski Z, Gilberger TW, Green JL, Holder AA, Cowman AF. A conserved molecular motor drives cell invasion and gliding motility across malaria life cycle stages and other apicomplexan parasites. J Biol Chem. 2006 281(8):5197-208.
See original on MMP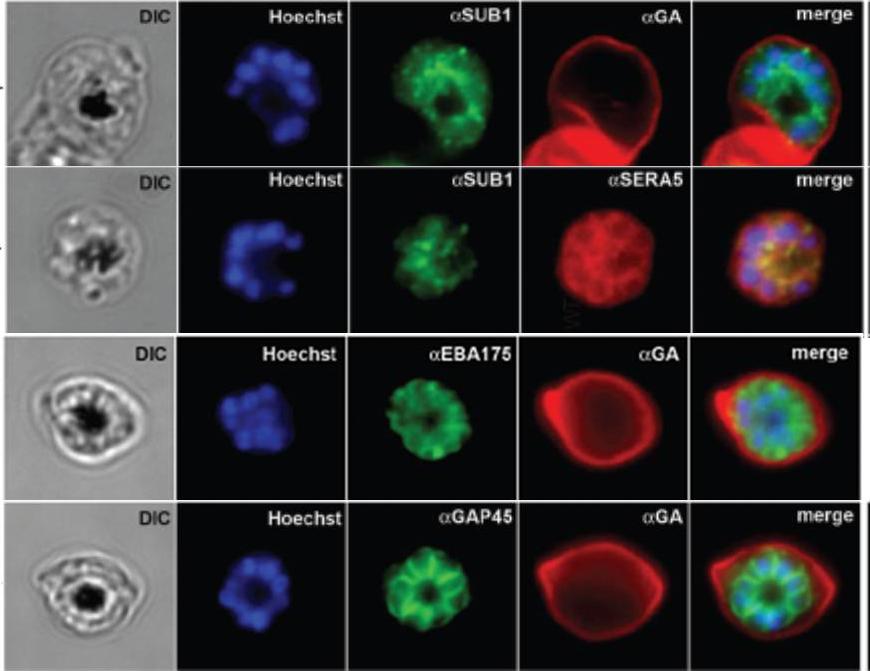
IFA of WT schizonts with glycophorin A (GA) labeled erythrocytes, localized SUB1 in an apical punctate pattern similar to SERA5. EBA-175 was detected in the apical ends of merozoites. GAP45 appeared submembrane. DIC, differential interference contrast; Hoechst = nucleic acid stain.Balu B, Maher SP, Pance A, Chauhan C, Naumov AV, Andrews RM, Ellis PD, Khan SM, Lin JW, Janse CJ, Rayner JC, Adams JH. CCR4-associated factor-1 coordinates expression of Plasmodium falciparum egress and invasion proteins. Eukaryot Cell. 2011 10(9):1257-63
See original on MMP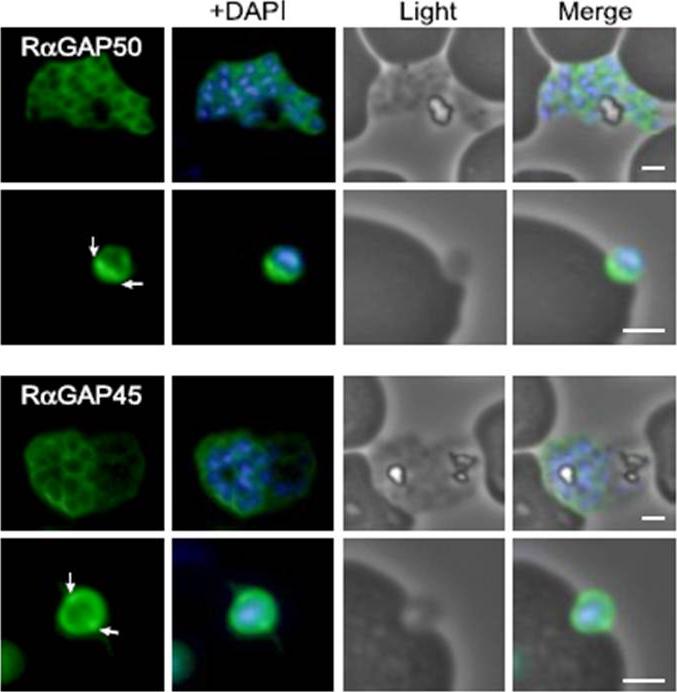
Characterization of PfGAP50 and PfGAP45 proteins. rabbit antiserum against recombinant PfGAP50 localizes to the merozoite periphery and remains following rupturing of schizont, consistent with localization to the IMC. The white arrows on putative invading merozoite point to interface with erythrocyte where increased fluorescence is detectable, possibly indicating concentration at the moving junction. Rabbit antiserum against recombinant PfGAP45 similarly suggests localization to the IMC. A second putative invading merozoite also suggests PfGAP45 is concentrated at the interface (white arrows) with the erythrocyte, consistent with the moving junction. Scale bar, 1 mm.Baum J, Richard D, Healer J, Rug M, Krnajski Z, Gilberger TW, Green JL, Holder AA, Cowman AF. A conserved molecular motor drives cell invasion and gliding motility across malaria life cycle stages and other apicomplexan parasites. J Biol Chem. 2006 281(8):5197-208.
See original on MMP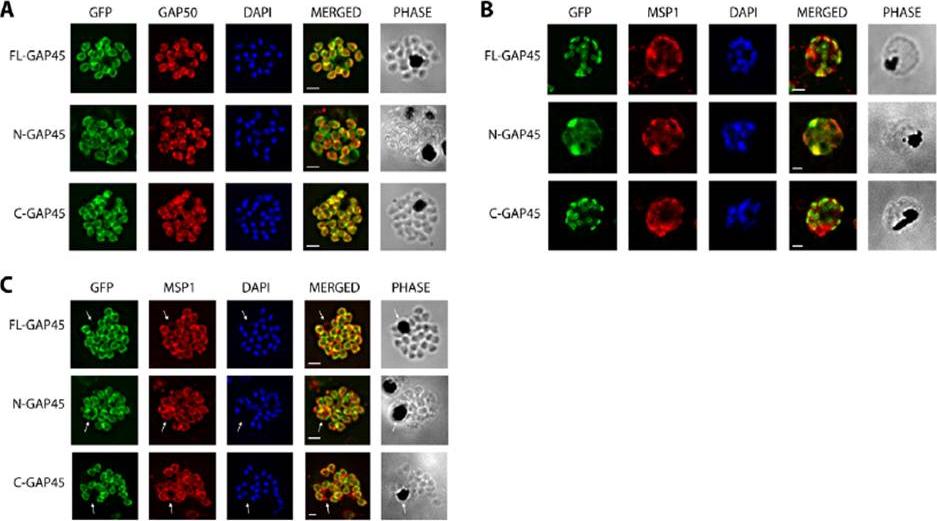
Immunofluorescent staining of GFP-tagged GAP45 variants with GAP50 in late stage schizonts. (A) Parasites were fixed and the location of GFP (green) and GAP50 (red), examined. FL-GAP45, C-GAP45 and N-GAP45 all appear to colocalise with GAP50 in segmented schizonts. (B) Staining with anti-MSP1 antibody (red) colocalises with only N-GFP in developing schizonts. FL-GAP45 AND C-GAP45 are present in distinctive ring-shaped structures at the periphery of the developing schizont. (C) In mature schizonts MSP1 (red) is present on membranes surrounding the residual body. N-GAP45 is also present on the residual body, but FL-GAP45 and C-GAP45 are absent. The white arrow in all images indicates the residual body. These data are consistent with a plasma membrane location for N-GAP45 and an inner membrane complex location for FL-GAP45. In all cases, parasite nuclei were stained with DAPI (blue); merged images are also shown. Scale bar is 2 mm.Immunofluorescent staining of GFP-tagged GAP45 variants with GAP50 in late stage schizonts. (A) Parasites were fixed and the location of GFP (green) and GAP50 (red), examined. FL-GAP45, C-GAP45 and N-GAP45 all appear to colocalise with GAP50 in segmented schizonts. (B) Staining with anti-MSP1 antibody (red) colocalises with only N-GFP in developing schizonts. FL-GAP45 AND C-GAP45 are present in distinctive ring-shaped structures at the periphery of the developing schizont. (C) In mature schizonts MSP1 (red) is present on membranes surrounding the residual body. N-GAP45 is also present on the residual body, but FL-GAP45 and C-GAP45 are absent. The white arrow in all images indicates the residual body. These data are consistent with a plasma membrane location for N-GAP45 and an inner membrane complex location for FL-GAP45. In all cases, parasite nuclei were stained with DAPI (blue); merged images are also shown. Scale bar is 2 mm.Ridzuan MA, Moon RW, Knuepfer E, Black S, Holder AA, Green JL. Subcellular Location, Phosphorylation and assembly into the motor complex of GAP45 during Plasmodium falciparum schizont development. PLoS One. 2012;7(3):e33845
See original on MMP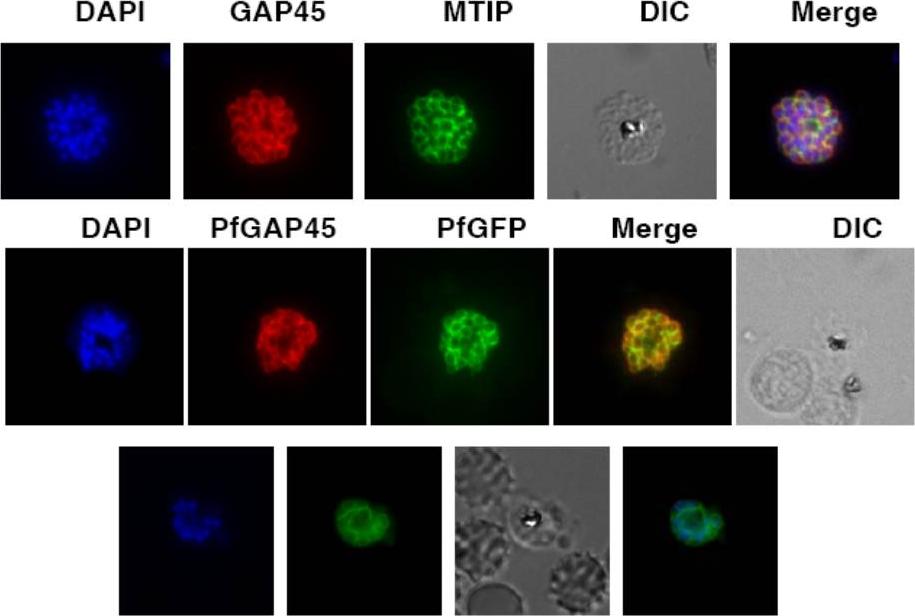
Upper row: PfGAP45 co-localizes with the IMC marker PfMTIP. Immunofluorescence assays were performed using anti-GFP (red) and anti-PfMTIP (green) antibodies on parasites over-expressing PfGAP45-GFP. PfGAP45 shows co-localization with PfMTIP around the periphery of merozoites in mature schizonts. Middle row: PfGAP45 phosphorylation mutants co-localize with endogenous PfGAP45. Immunofluorescence assays were performed using anti-GFP (green) and anti-PfGAP45 (red) antibodies to localize episomally expressing GAP45-GFP and endogenous PfGAP45.Lower row: Live Imaging of PfGAP45 GFP in parasites over-expressing PfGAP45-GFP was performed after labeling the nuclei with DAPI. Thomas DC, Ahmed A, Gilberger TW, Sharma P. Regulation of Plasmodium falciparum Glideosome Associated Protein 45 (PfGAP45) Phosphorylation. PLoS One. 2012;7(4):e35855.
See original on MMP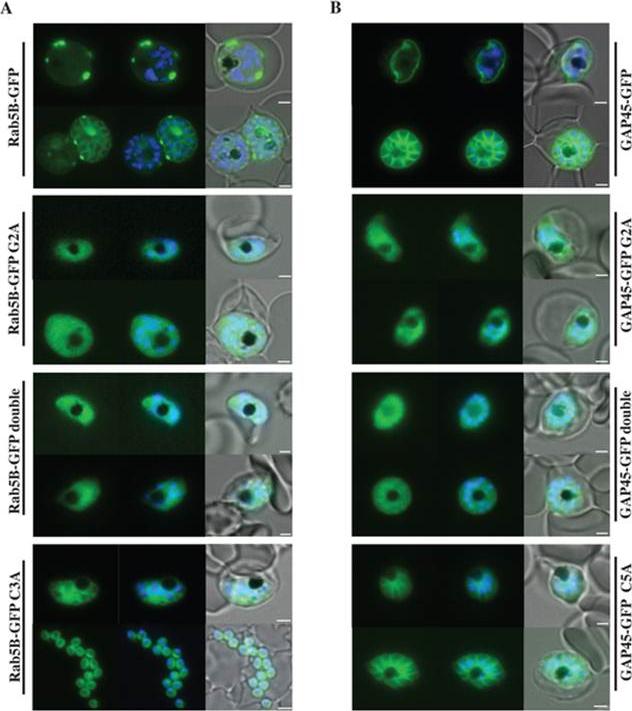
Localisation of PfRab5B-GFP fusion proteins in P. falciparum. Parasites were transfected with constructs expressing the N-terminal 28 amino acids of PfRab5B or 29 amino acids of PfGAP45 fused to GFP under a schizont stage-specific promoter (msp3 59UTR region). Localisation of PfRab5B28-GFP (A) and GAP4529-GFP fusions (B) as well as myristoylation (G2A) and palmitoylation-deficient fusions (C3A in PfRab5B28-GFP and C5A in GAP4529-GFP) were investigated. The first image in a series corresponds to GFP fluorescence, the second a merge of GFP fluorescence with nuclear DAPI stain, and the third a merge of GFP fluorescence, DAPI and bright field images. Size bars are 2 mm. GFP was observed at the plasma membrane (top right hand panel). In transgenic parasites that expressed GFP fused to G2A variants of both PfRab5B and GAP45 the association of GFP with the plasma membrane was lost and we observed a diffuse GFP-staining throughout the cytoplasm of the parasites .Ezougou CN, Ben-Rached F, Moss DK, Lin JW, Black S, Knuepfer E, Green JL, Khan SM, Mukhopadhyay A, Janse CJ, Coppens I, Yera H, Holder AA, Langsley G. Plasmodium falciparum Rab5B Is an N-Terminally Myristoylated Rab GTPase That Is Targeted to the Parasite's Plasma and Food Vacuole Membranes. PLoS One. 2014 9(2):e87695.
See original on MMP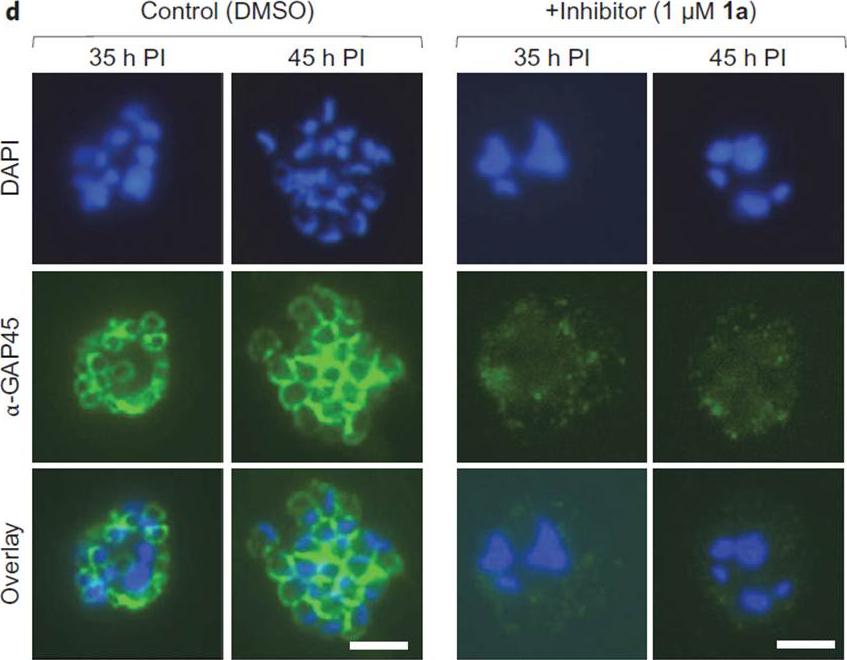
N-Myristoyltransferase (NMT) inhibition results in loss of the IMC and irreversible loss of parasite viability before parasite egress. d, Indirect immunofluorescence microscopy of parasites 35 h or 45 h PI, cultured in the presence of NMT inhibitor (1 mM 1a) or DMSO. Green, secondary antibody fluorescence; blue, DAPI (nuclear stain). All images at identical magnification. Scale bars, 5 mm. In Plasmodium, nuclear division precedes cell division, and the IMC initially appears as small ring-like structures, which then extend around developing merozoites during schizont segmentation (cell division). This transition was recapitulated in immunofluorescence images of DMSO-treated parasites at 35 and 45 h PI, whereas in the inhibitor treated parasites the IMC structure was not visible at either time point.Wright MH, Clough B, Rackham MD, Rangachari K, Brannigan JA, Grainger M, Moss DK, Bottrill AR, Heal WP, Broncel M, Serwa RA, Brady D, Mann DJ, Leatherbarrow RJ, Tewari R, Wilkinson AJ, Holder AA, Tate EW. Validation ofN-myristoyltransferase as an antimalarial drug target using an integratedchemical biology approach. Nat Chem. 2014 6(2):112-21.
See original on MMP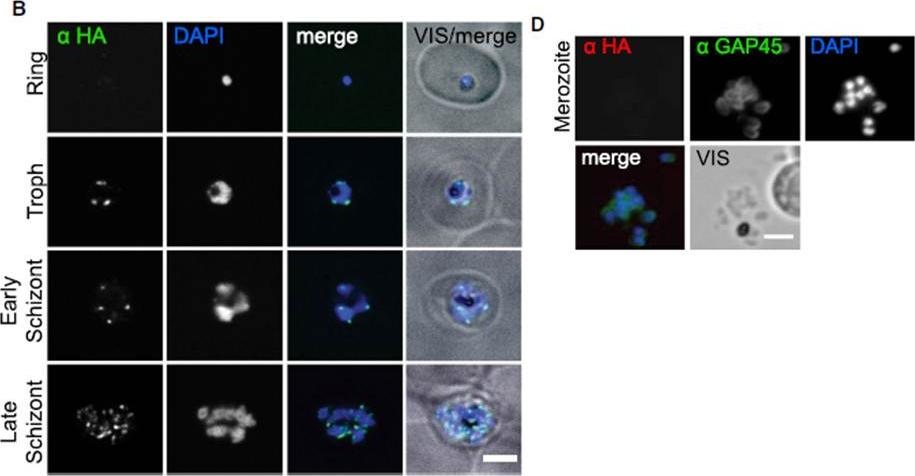
PfHda2 Is an Essential Nuclear Histone Deacetylase. (B) Immunofluorescence detection of PfHda2-HA in the perinuclear region of late stage asexual P. falciparum. Scale bar, 3 mm. (D) Immunofluorescence did not detect PfHda2-HA in merozoites costained with anti-GAP45.Coleman BI, Skillman KM, Jiang RH, Childs LM, Altenhofen LM, Ganter M, Leung Y, Goldowitz I, Kafsack BF, Marti M, Llinás M, Buckee CO, Duraisingh MT. A Plasmodium falciparum Histone Deacetylase Regulates Antigenic Variation and Gametocyte Conversion. Cell Host Microbe. 2014 16(2):177-86. PMID:
See original on MMP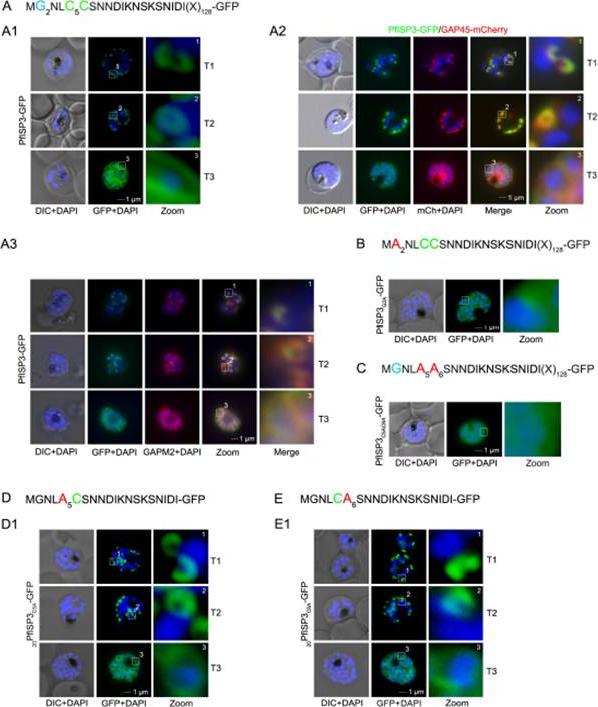
The role of N-terminal acylation for IMC membrane association of PfISP3. A. Over-expression of PfISP3-GFP showing characteristic IMC dynamics during schizogony (T1-T3) in unfixed parasites. Nuclei are stained with DAPI (blue). Enlargement of selected areas are marked with a white square and referred to as zoom. Scale bar, 1 μm (A1). A2. Co-localization of PfISP3-GFP (green) and GAP45mCherry (red) in unfixed parasites revealed their identical dynamic during schizogony. A3. Colocalization with the IMC marker GAPM2 (α-GAPM2, red) in fixed cells. Putative myristoylation and palmitoylation sites are highlighted in light blue (G2) or green (C5C6). B-C. IMC membrane association depends on the presence of N-terminal myristoylation and palmitoylation motifs. N-terminal myristoylation (B, PfISP3G2A-GFP) and palmitoylation motif mutants (C, PfISP3C5AC6A-GFP) were expressed in P. falciparum and localized in unfixed parasites. Mutation of either the myristoylation or the palmitoylation motifs resulted in a cytosolic distribution of the GFP-fusion protein (B1, C1). D-E. Mutation of the individual palmitoylation sites C5 (PfISP3C5A-GFP) or C6 (PfISP3C6A-GFP) does not interfere with IMC recruitment of the fusion protein. Wetzel J, Herrmann S, Swapna LS, Prusty D, Peter AT, Kono M, Saini S, Nellimarla S, Wong TW, Wilcke L, Ramsay O, Cabrera A, Biller L, Heincke D, Mossman K, Spielmann T, Ungermann C, Parkinson J, Gilberger TW. The role of palmitoylation for protein recruitment to the inner membrane complex of the malaria parasite. J Biol Chem. 2014 Nov 25.
See original on MMP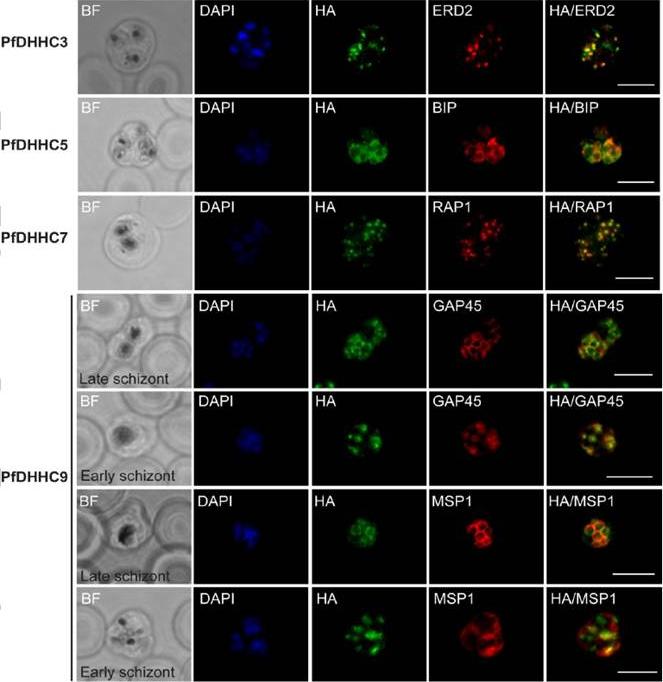
Expression and localisation of PfDHHC proteins in P. falciparum schizonts. Triple-HA-tagged PfDHHC proteins were localised by immunofluorescence using antibodies against the 3-HA tag (green). Immunofluorescence staining of each of the tagged PfDHHC proteins was compared against that of the following known localisation markers (red): ERD2 (Golgi marker), BIP (endoplasmic reticulum marker), RAP1 (rhoptry marker), GAP45 (inner membrane complex marker) and MSP1 (plasma membrane marker). Nuclear staining by DAPI is shown in blue. For the staining of PfDHHC9 with GAP45 and MSP1, both a late schizont, as well as an early schizont, is shown in order to differentiate between IMC and plasma membrane localisation. Scale bar: 5 μm.Tay CL, Jones ML, Hodson N, Theron M, Choudhary JS, Rayner JC. Study of Plasmodium falciparum DHHC palmitoyl-transferases identifies a role for PfDHHC9 in gametocytogenesis. Cell Microbiol. 2016 Apr 6.
See original on MMP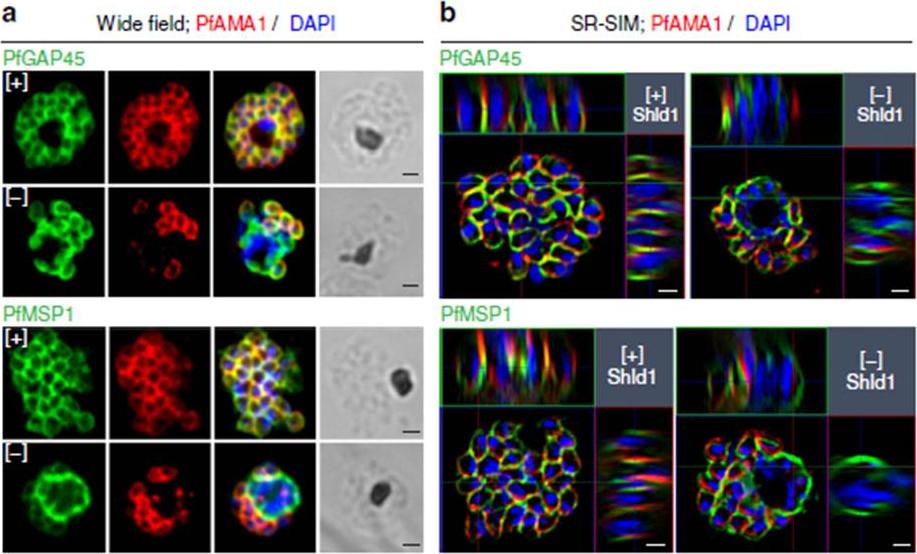
PfMOP deficiency leads to incomplete formation of the IMC. (a) Wide-field and (b) SR-SIM (super-resolution structured illumination microscopy) of representative pictures of E64-treated [+]/[-] Shld1 schizont stage PfMOP-DD parasites using antibodies against PfGAP45 and PfMSP1. Scale bar, 1 mm.Absalon S, Robbins JA, Dvorin JD. An essential malaria protein defines the architecture of blood-stage and transmission-stage parasites. Nat Commun. 2016 7:11449.
See original on MMP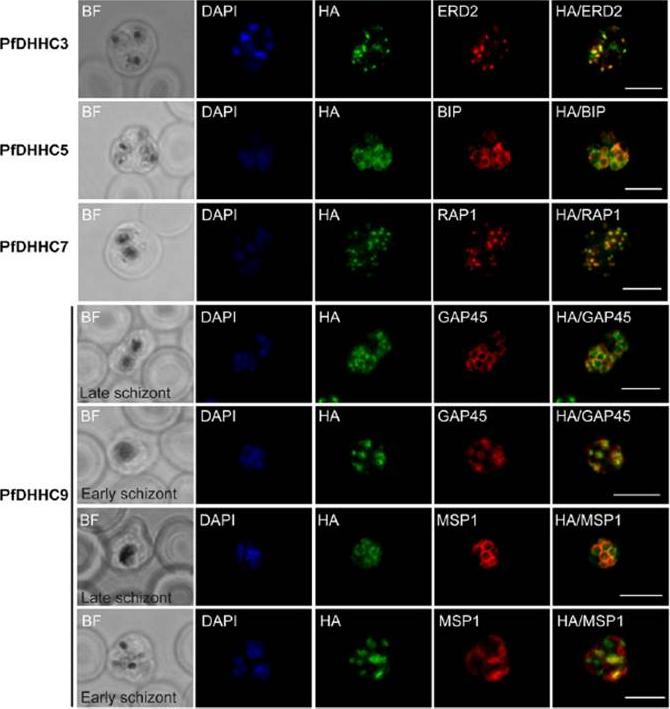
Expression and localization of PfDHHC proteins in Plasmodium falciparum schizonts. Triple-HA-tagged PfDHHC proteins were localized by immuno-fluorescence using antibodies against the 3-HA tag (green). Immunofluorescence staining of each of the tagged PfDHHC proteins was compared against that of the following known localization markers (red): ERD2 (Golgi marker), BIP (endoplasmic reticulum marker), RAP1 (rhoptry marker), GAP45 (inner membrane complex marker) and MSP1 (plasma membrane marker). Nuclear staining by DAPI is shown in blue. For the staining of PfDHHC9 with GAP45 and MSP1, a late schizont, as well as an early schizont, is shown in order to differentiate between inner membrane complex and plasma membrane localization. Scale bar: 5 μm.Tay CL, Jones ML, Hodson N, Theron M, Choudhary JS, Rayner JC. Study of Plasmodium falciparum DHHC palmitoyl transferases identifies a role for PfDHHC9 in gametocytogenesis. Cell Microbiol. 2016 18(11):1596-1610.
See original on MMP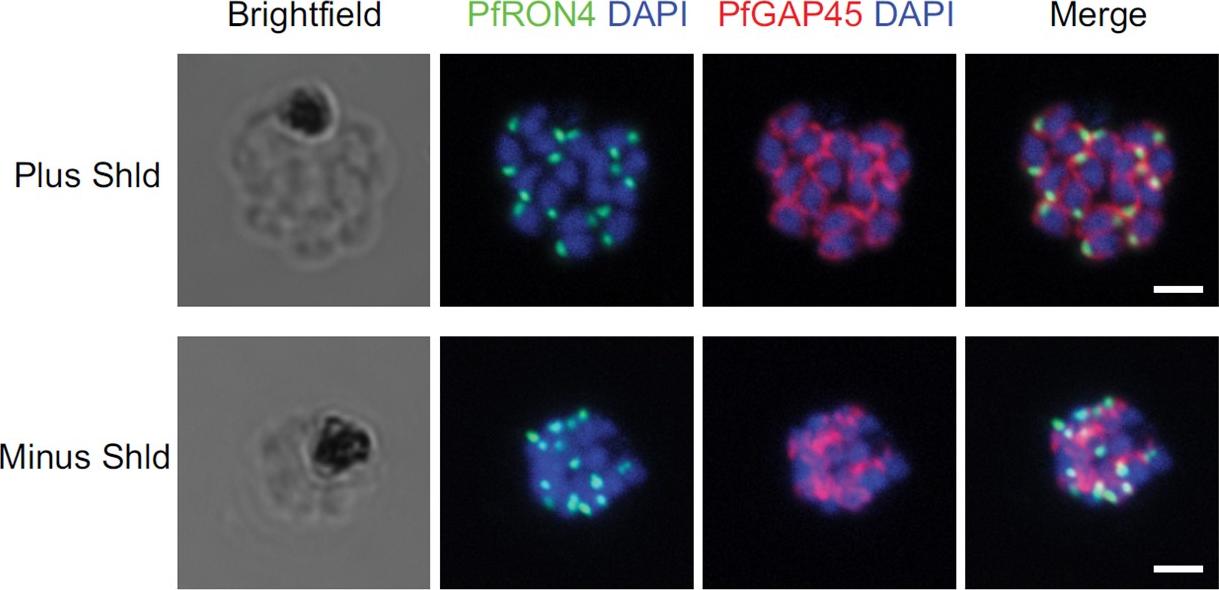
IFA of parasites grown in the presence and absence of Shld probed with antibodies against PfRON4 (green) and PfGAP45 (red). Bars, 2 mm. Staining for PfGAP45, a soluble protein that complexes with PfGAP50 (along with PfMyoA and PfMTIP) to localize to the inner membrane complex (IMC), revealed readily detected expression of the PfGAP45 protein but the absence of normal morphology in PfCyc1-deficient parasites. In contrast, IFAs against the rhoptry protein PfRON4 suggests unencumbered rhoptry formation. Similarly, IFA against the In contrast, IFAs against the rhoptry protein PfRON4 suggests unencumbered rhoptry formation.Robbins JA, Absalon S, Streva VA, Dvorin JD. The Malaria Parasite Cyclin H Homolog PfCyc1 Is Required for Efficient Cytokinesis in Blood-Stage Plasmodium falciparum. MBio. 2017 Jun 13;8(3). pii: e00605-17.
See original on MMP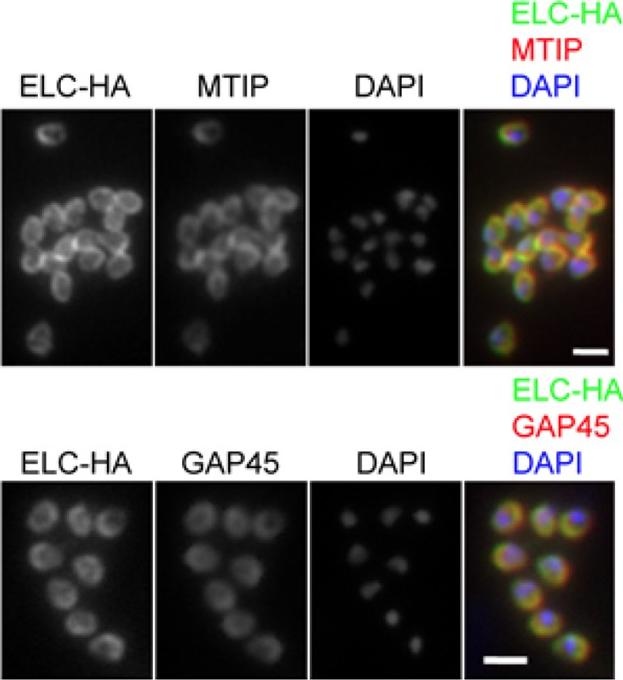
PfELC expression matches that of known glideosome components in P. falciparum schizonts. Immunofluorescent detection of HA-tagged ELC and either MTIP or GAP45 in mature schizonts and merozoites. Merged colour images are also shown, with ELC-HA (green) and MTIP or GAP45 (red). Nuclei are stained with DAPI (blue). Scale bar: 2 μm. Immunofluorescence analysis of fixed blood-stage P. falciparum with HA-specific antibodies showed co-localisation of the protein with GAP45 and MTIP in late, segmented schizonts.Green JL, Wall RJ, Vahokoski J, Yusuf NA, Ridzuan MAM, Stanway RR, Stock J, Knuepfer E, Brady D, Martin SR, Howell SA, Pires IP, Moon RW, Molloy JE, Kursula I, Tewari R, Holder AA. Compositional and expression analyses of the glideosome during the Plasmodium life cycle reveal an additional myosin light chain required for maximum motility. J Biol Chem. 2017 Sep 11.
See original on MMP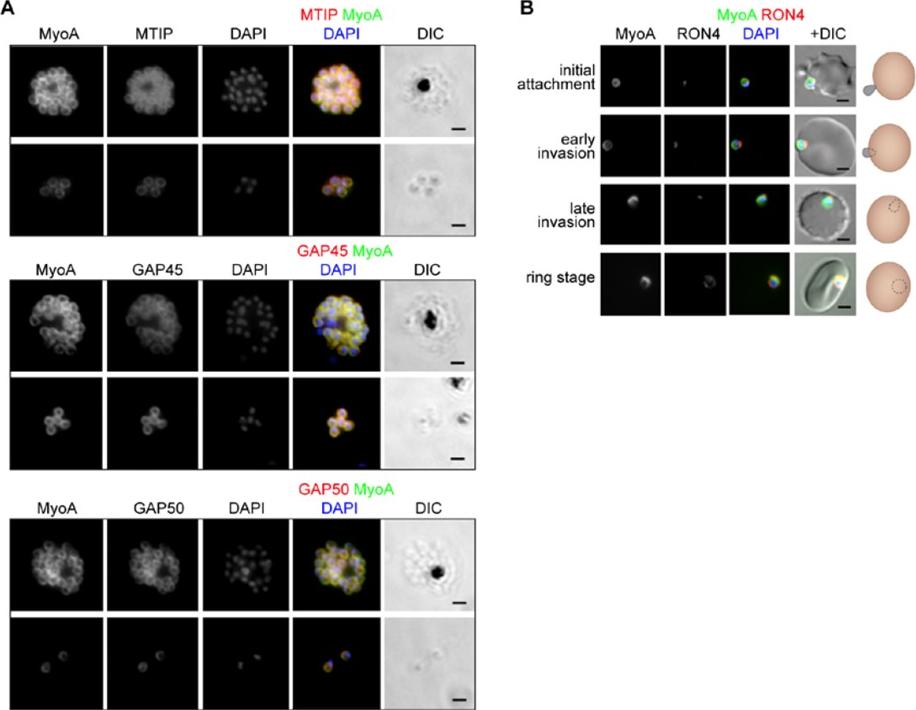
The location of MyoA during P. falciparum merozoite invasion. (A) MyoA-GFP is located at the periphery of developing intracellular (top row of each pair of images) and free extracellular merozoites (bottom row), and is colocalized with antibodies specific for IMC proteins MTIP, GAP45 and GAP50. In the merged colour image the MyoA-GFP signal is green and antibodies specific for the IMC proteins are red; nuclei are stained blue with DAPI. The DIC image is also shown. (B) Individual merozoites are captured at different stages of invasion from initial attachment, through early and late invasion to the intracellular ring stage. MyoA-GFP remains peripheral whereas RON4, initially in the apical rhoptry neck, relocates during invasion. Merged colour images with MyoA-GFP (green), RON4 (red), and nuclei (blue) and DIC images are also shown, together with a schematic of each cell-pair. Scale bar: 2 μm.Green JL, Wall RJ, Vahokoski J, Yusuf NA, Ridzuan MAM, Stanway RR, Stock J, Knuepfer E, Brady D, Martin SR, Howell SA, Pires IP, Moon RW, Molloy JE, Kursula I, Tewari R, Holder AA. Compositional and expression analyses of the glideosome during the Plasmodium life cycle reveal an additional myosin light chain required for maximum motility. J Biol Chem. 2017 Sep 11.
See original on MMPMore information
| PlasmoDB | PVP01_1440900 |
| GeneDB | PVP01_1440900 |
| Malaria Metabolic Pathways | Localisation images Pathways mapped to |
| Previous ID(s) | null |
| Orthologs | PBANKA_1437600 , PCHAS_1439600 , PF3D7_1222700 , PKNH_1441800 , PVX_123765 , PY17X_1440100 |
| Google Scholar | Search for all mentions of this gene |