PF3D7_1460100 FYVE and coiled-coil domain-containing protein (FCP)
Disruptability [+]
| Species | Disruptability | Reference | Submitter |
|---|---|---|---|
| P. falciparum 3D7 |
Refractory |
USF piggyBac screen (Insert. mut.) | USF PiggyBac Screen |
Mutant phenotypes [+]
None reported yet. Please press the '+' button above to add one.Imaging data (from Malaria Metabolic Pathways)
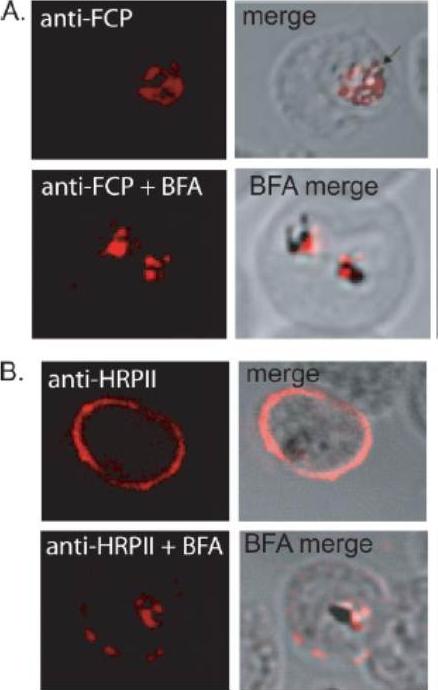
FCP localizes to the FV but does not traffic via the classical or alternate secretory pathways. A, immunofluorescence using anti-FCP revealed a discrete localization to the parasite FV. Ring stage parasites were treated either with 5 mg/ml BFA (anti-FCPP+BFA) or solvent as control (anti-FCP) for 12 h before immunofluorescence microscopy. BFA treatment did not alter FV localization of FCP. B, as a control experiment, parasites treated with BFA (anti-HRPIIBFA) or without BFA (anti-HRPII) were stained using antisera against a known secreted protein, HRPII. HRPII was localized predominantly to the cytoplasm of the host infected erythrocyte (anti-HRPII). BFA treatment resulted in a significant loss in erythrocyte localization of HRPII and a concomitant increase in the parasite cytoplasm (anti-HRPII + BFA).McIntosh MT, Vaid A, Hosgood HD, Vijay J, Bhattacharya A, Sahani MH, Baevova P, Joiner KA, Sharma P. Traffic to the malaria parasite food vacuole: a novel pathway involving a phosphatidylinositol 3-phosphate-binding protein. J Biol Chem. 2007 282:11499-508.
See original on MMP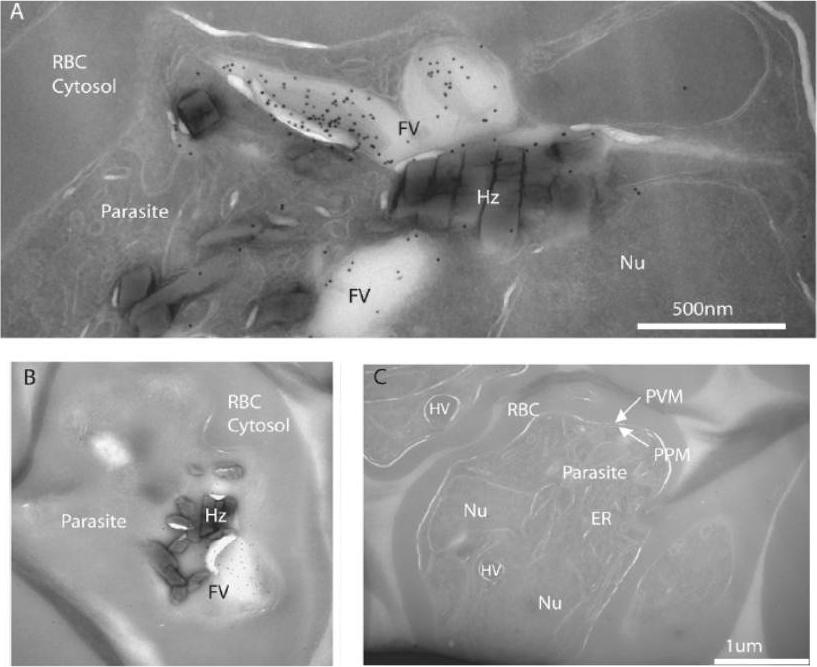
FCP localizes to the lumen of the FV. A, cryo-immunoelectron micrograph showing a close-up of a folded FV with immunogold-stained FCP in the lumen of the parasite FV in a late trophozoite. Various structures are indicated as host red blood cell cytosol (RBC Cytosol), parasite body (parasite), parasite nucleus (Nu), FV, and hemozoin (Hz). B shows a more complete parasite with a cross-section through the FV indicating a lack of immunogold staining outside the FV. C, three parasites from a different region of the same ultrathin section that was immunogold-stained for anti-FCP in A and B. Note the lack of concentrated gold particles on all visible parasite structures including ER, parasite plasma membrane (PPM), parasitophorous vacuolar membrane (PVM), and hemoglobin containing vesicles (HV). McIntosh MT, Vaid A, Hosgood HD, Vijay J, Bhattacharya A, Sahani MH, Baevova P, Joiner KA, Sharma P. Traffic to the malaria parasite food vacuole: a novel pathway involving a phosphatidylinositol 3-phosphate-binding protein. J Biol Chem. 2007 282:11499-508.
See original on MMP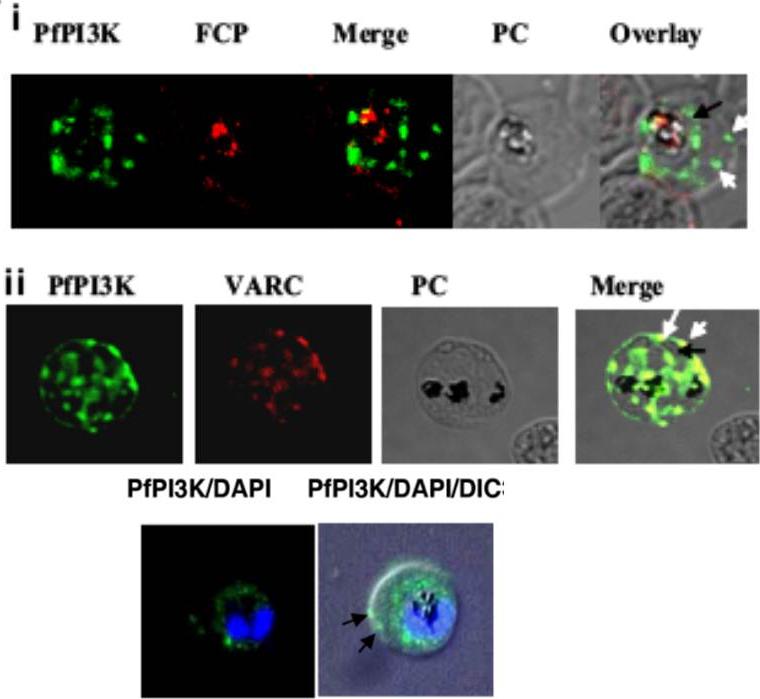
Intracellular localization of PfPI3K and its trafficking to the host. (A) IFA was performed for localizing PfPI3K, FCP (i) and VARC (ii). FCP (red) is located inside the food vacuole of mature trophozoites. PfPI3K (green) exhibits “vesicular” staining on PVM/PM (black arrows) and the host erythrocyte (white arrows) and in the food vacuole, which can be identified by the presence of black hemozoin. (ii) PfPI3K colocalizes with VARC at the erythrocyte surface. PfPI3K localization. Lower row: Early trophozoites on thin blood smears were used for IFA to assess the localization of PfPI3K (green). The parasite nuclei (blue) are stained with DAPI. The arrows indicate PfPI3K near the host RBC membrane.Vaid A, Ranjan R, Smythe WA, Hoppe HC, Sharma P. PfPI3K, a phosphatidylinositol-3 kinase from Plasmodium falciparum, is exported to the host erythrocyte and is involved in hemoglobin trafficking. Blood. 2010 115:2500-2507.
See original on MMP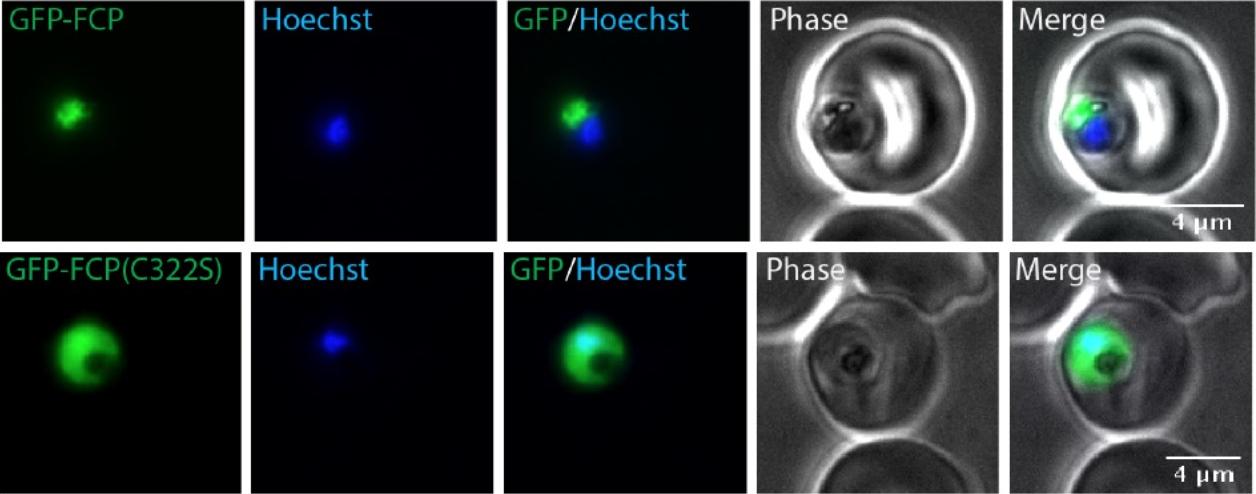
A CaaX motif is required for FCP localization. Live epifluorescent images of P. falciparum parasites expressing either Wt GFP-FCP (top panel) or GFP-FCP(C322S) (bottom panel). The nucleus was stained using Hoechst. GFP-FCP showed fluorescence overlapping or surrounding parasite hemozoin, consistent with its previously reported food vacuole localization. However, GFP-FCP(C322S) fluorescence appeared throughout the cell consistent with cytosolic mislocalization.Gisselberg JE, Zhang L, Elias JE, Yeh E. The prenylated proteome of Plasmodium falciparum reveals pathogen-specific prenylation activity and drug mechanism-of-action. Mol Cell Proteomics. 2016 Dec 31. pii: mcp.M116.064550. PMID: 28040698.
See original on MMP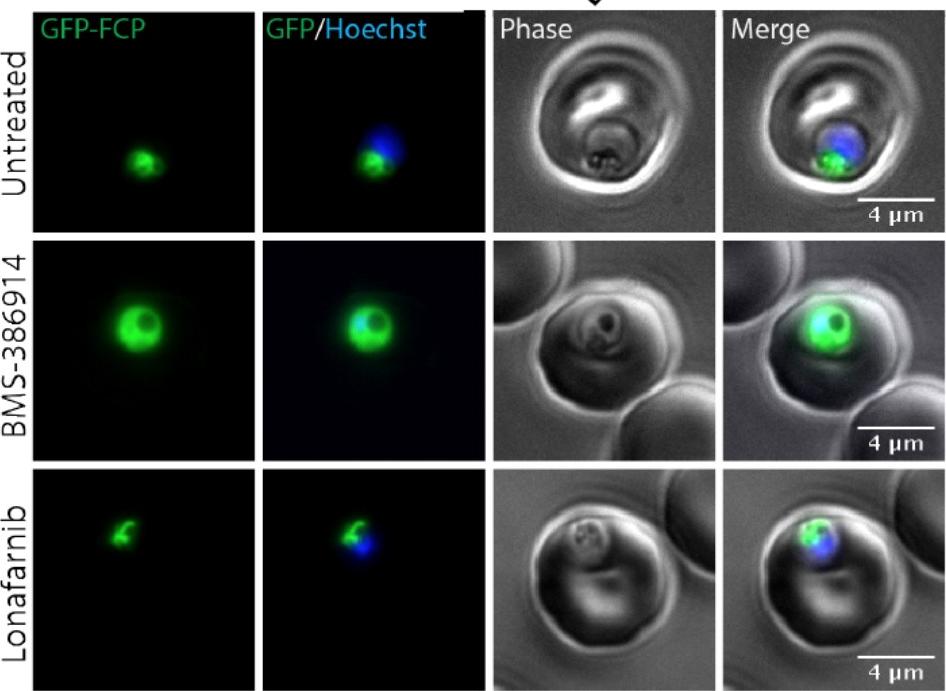
BMS-386914 inhibits FCP prenylation and causes mislocalization to the cytosol. Live microscopy of GFP-FCP expressing parasites treated with10x EC50 BMS-386914 or lonafarnib. The nucleus was stained using Hoechst. In contrast, lonafarnib showed no effect on FCP prenylation or localization even at 10x EC50 concentrations (Figure 5B and 5C). These results show that BMS-386914 inhibits prenylation and proper localization of FCP as part of its antimalarial mechanism-of-action.Gisselberg JE, Zhang L, Elias JE, Yeh E. The prenylated proteome of Plasmodium falciparum reveals pathogen-specific prenylation activity and drug mechanism-of-action. Mol Cell Proteomics. 2016 Dec 31. pii: mcp.M116.064550.
See original on MMPMore information
| PlasmoDB | PF3D7_1460100 |
| GeneDB | PF3D7_1460100 |
| Malaria Metabolic Pathways | Localisation images Pathways mapped to |
| Previous ID(s) | PF14_0574 |
| Orthologs | PBANKA_1323700 , PCHAS_1327000 , PKNH_1221700 , PVP01_1246800 , PVX_117455 , PY17X_1327500 |
| Google Scholar | Search for all mentions of this gene |