PF3D7_1452000 rhoptry neck protein 2 (RON2)
Disruptability [+]
| Species | Disruptability | Reference | Submitter | |
|---|---|---|---|---|
| P. falciparum 3D7 |
Refractory |
USF piggyBac screen (Insert. mut.) | USF PiggyBac Screen | |
| P. berghei ANKA |
Refractory |
RMgm-214 | Imported from RMgmDB | |
Mutant phenotypes [+]
None reported yet. Please press the '+' button above to add one.Imaging data (from Malaria Metabolic Pathways)
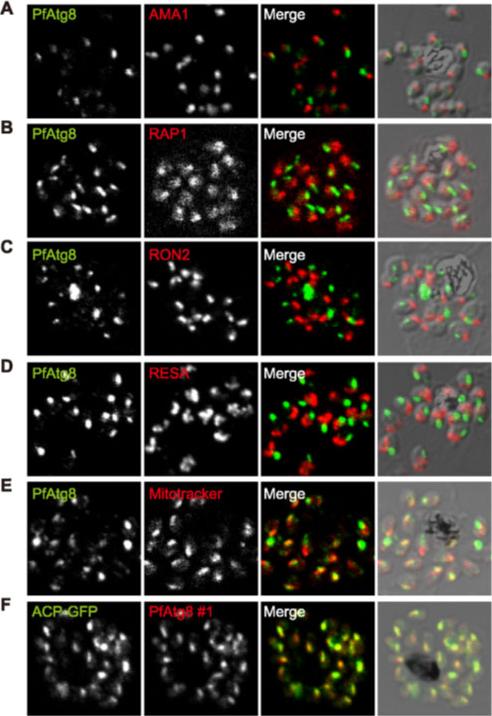
PfAtg8 localizes to the apicoplast. P. falciparum FCR3 (A–E) and P. falciparum 3D7 transfected with ACP-GFP (F–H) were stained with the indicated organelle markers and visualized by confocal microscopy (because ACP-GFP was not uniformly expressed, some merozoites displayed only faint GFP signals). Anti-PfAtg8 antibody #1 was used in (A–F), and anti-PfAtg8 antibody #2 was used in (G). Apical membrane antigen 1 (AMA1) as a microneme marker (A), rhoptry-associated protein 1 (RAP1) as a rhoptry body marker (B), rhoptry neck protein 2 (RON2) as a rhoptry neck marker (C), the ring-infected erythrocyte surface antigen (RESA) as a dense granule marker (D), MitoTrackerRed CMXRos as a mitochondria marker (E), ACPGFP (F–H) and the organellar histone-like protein PfHU (H) as an apicoplast marker were used. Scale bar, 1 mm.Kitamura K, Kishi-Itakura C, Tsuboi T, Sato S, Kita K, Ohta N, Mizushima N. Autophagy-Related Atg8 Localizes to the Apicoplast of the Human Malaria Parasite Plasmodium falciparum. PLoS One. 2012;7(8):e42977.
See original on MMP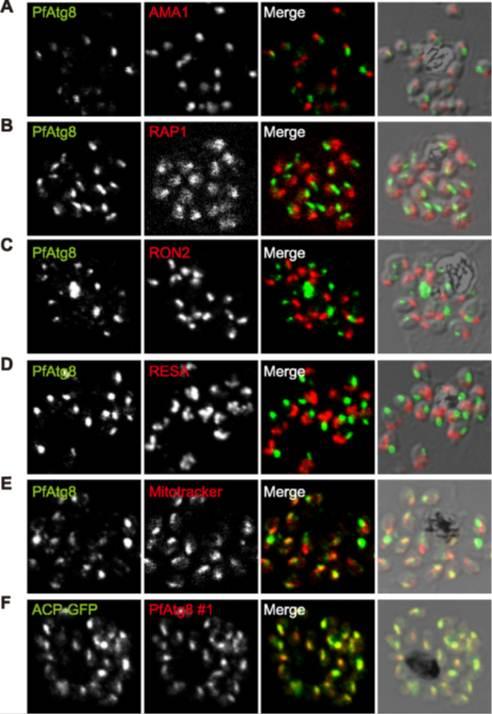
PfAtg8 localizes to the apicoplast. P. falciparum FCR3 (A–E) and P. falciparum 3D7 transfected with ACP-GFP (F–H) were stained with the indicated organelle markers and visualized by confocal microscopy (because ACP-GFP was not uniformly expressed, some merozoites displayed only faint GFP signals). Anti-PfAtg8 antibody #1 was used in (A–F), and anti-PfAtg8 antibody #2 was used in (G). Apical membrane antigen 1 (AMA1) as a microneme marker (A), rhoptry-associated protein 1 (RAP1) as a rhoptry body marker (B), rhoptry neck protein 2 (RON2) as a rhoptry neck marker (C), the ring-infected erythrocyte surface antigen (RESA) as a dense granule marker (D), MitoTrackerRed CMXRos as a mitochondria marker (E), ACPGFP (F–H) and the organellar histone-like protein PfHU (H) as an apicoplast marker were used. Scale bar, 1 mm.Kitamura K, Kishi-Itakura C, Tsuboi T, Sato S, Kita K, Ohta N, Mizushima N. Autophagy-Related Atg8 Localizes to the Apicoplast of the Human Malaria Parasite Plasmodium falciparum. PLoS One. 2012;7(8):e42977
See original on MMP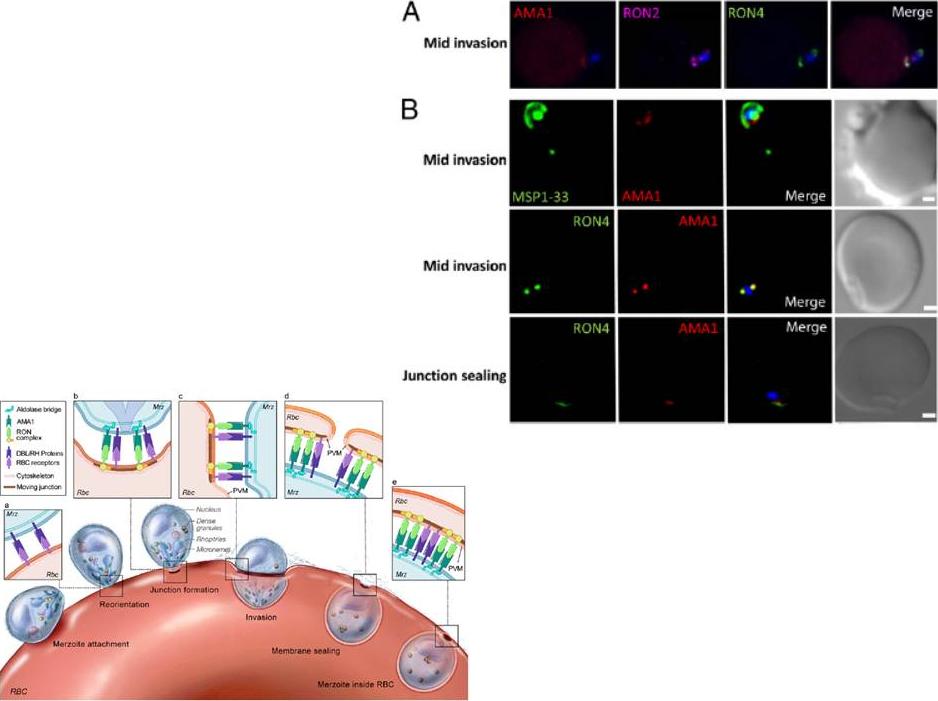
Localization of Pf AMA1, RON2, and RON4 at the moving junction of invading merozoites. Confocal microscopy images of merozoite invading RBC are shown. (A) Pf AMA1, RON2, and RON4 colocalize during invasion. (B) AMA1 is present at the moving junction (Top) with RON4 colocalizing with AMA1 at the junction (Middle). RON4 marks the sealing of the junction after invasion (Bottom). Scale bars represent 1μM.Schematic model of the steps involved in Pf merozoite (Mrz) invasion.Srinivasan P, Beatty WL, Diouf A, Herrera R, Ambroggio X, Moch JK, Tyler JS, Narum DL, Pierce SK, Boothroyd JC, Haynes JD, Miller LH. Binding of Plasmodium merozoite proteins RON2 and AMA1 triggers commitment to invasion. Proc Natl Acad Sci U S A. 2011 108(32):13275-80
See original on MMP
Left: PfRON2 is expressed at the apical end of Plasmodium merozoites. Schizont infected erythrocytes and merozoites were dual-labeled with antisera against PfRON2 and PfAMA1 PF11_0344 (A), PfClag3.1 PFC0110w (B), or PfRON4 PF11_0168 (C). Merged images are shown in the right panels. All segmented schizonts and merozoites are positive for PfRON2. Nuclei are counterstained with DAPI. Colocalization of PfRON2 with PfRON4 (rhoptry neck marker) was observed but neither colocalized with PfClag3.1 (rhoptry body marker) nor PfAMA1 (microneme marker). Right: Longitudinally sectioned merozoites in schizont-infected erythrocytes were labeled with anti-PfRON2 serum followed by secondary Ab conjugated with gold particles. Gold particles were restricted to the narrow neck portion of the rhoptries (R). Two different images are shown (A and B). N indicates nucleus. Bars=200 nm. Cao J, Kaneko O, Thongkukiatkul A, Tachibana M, Otsuki H, Gao Q, Tsuboi T, Torii M. Rhoptry neck protein RON2 forms a complex with microneme protein AMA1 in Plasmodium falciparum merozoites. Parasitol Int. 2009 58:29-35.
See original on MMP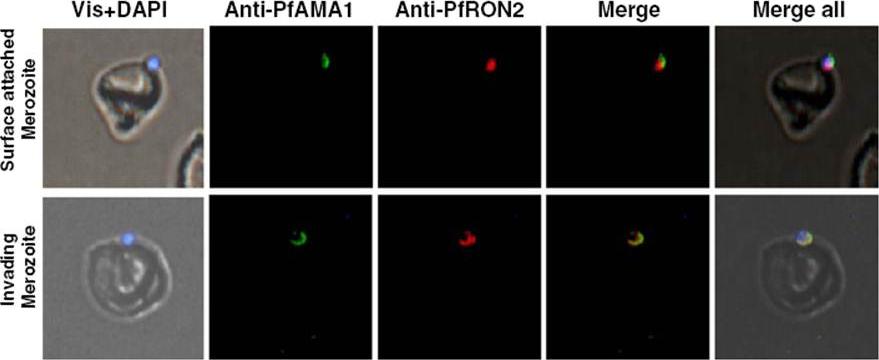
Immunofluorescence assay showing co-localization of PfRON2 with PfAMA1 at apical end at the time of meozoite attachment during merozoite invasion. At the time of attachment of the merozoite with the host RBCs, the PfRON2 staining was at the apical tip, the PfAMA1 staining was also mainly at the apical tip and with some distribution at the merozotie surface. During parasite invasion, the PfRON2 and PfAMA1 showed col-ocalization at the apical end of merozoite.Hossain ME, Dhawan S, Mohmmed A. The cysteine-rich regions of Plasmodium falciparum RON2 bind with host erythrocyte and AMA1 during merozoite invasion. Parasitol Res. 2011 110(5):1711-21
See original on MMP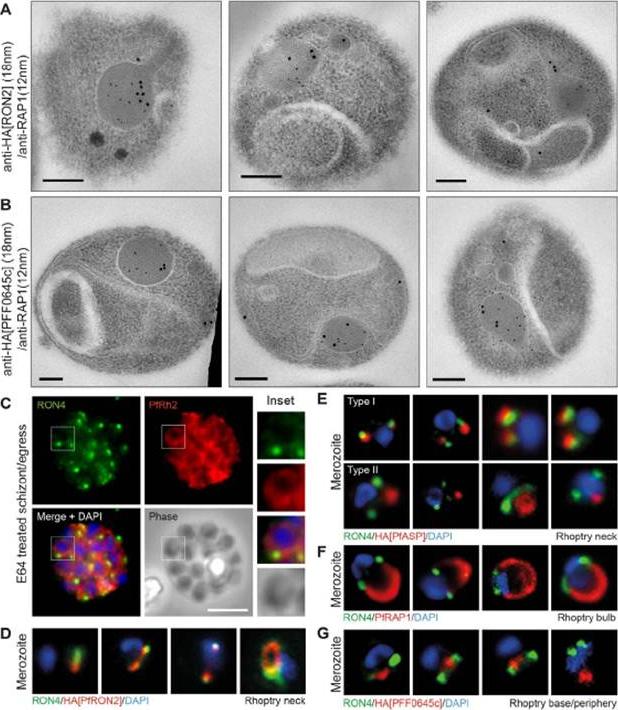
Spatial localisation of different rhoptry proteins before and during merozoite invasion. (A) IEM of free PfRON2-HA merozoites (pre-invasion) dual labeled with immunogold anti-HA (18 nm) and rhoptry bulb marker RAP1 (12 nm). Scale bar = 0.2 mm. (B) IEM of free PFF0645c-HA merozoites (pre-invasion) dual labeled with immunogold anti-HA (18 nm) and rhoptry bulb marker RAP1 (12 nm). Scale bar = 0.2 mm. (C) Widefield IFA of E64-treated schizonts (to prevent egress labeled with anti-PfRh2, anti-PfRON4 and DAPI. Scale bar = 5 mm. (D–G) Independent replicate imaging of merozoites from (D) PfRON2-HA, (E) PfASP-HA (two classes of distribution seen), (F) RAP1 and (G) PFF0645c-HA mid-way through invasion colabeled with anti-PfRON4 and DAPI. RON2-HA is located anterior to the bulb marker RAP1. PFF0645c had a more posterior or contiguous localisation to RAP1, frequently located towards the peripheral regions of the rhoptry.Zuccala ES, Gout AM, Dekiwadia C, Marapana DS, Angrisano F, Turnbull L, Riglar DT, Rogers KL, Whitchurch CB, Ralph SA, Speed TP, Baum J. Subcompartmentalisation of Proteins in the Rhoptries Correlates with Ordered Events of Erythrocyte Invasion by the Blood Stage Malaria Parasite. PLoS One. 2012;7(9):e46160.
See original on MMP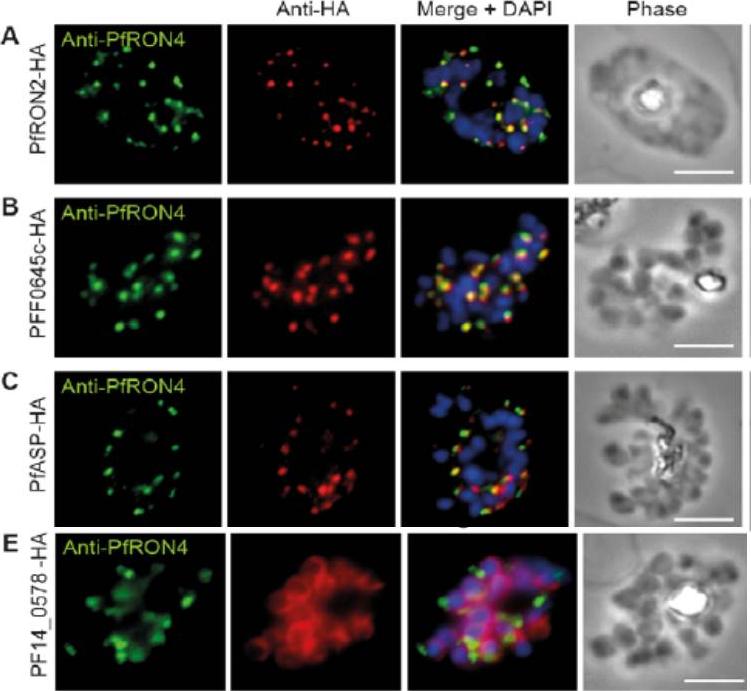
Cellular localisation of invasins in P. falciparum schizonts. (A–E) IFA of schizonts from HA tagged P. falciparum parasite lines labeled with anti-PfRON4, to mark the rhoptry neck, DAPI, to mark nuclei and anti-HA. (A) PfRON2 (B) rhoptry protein PFF0645c (C) apical sushi protein PfASP and (E) inner membrane complex protein PF14_0578. PfRON2-HA schizonts were colabeled with anti-PfRON4, a marker of the rhoptry neck. PFF0645c and PfASP each displayed a similar apical localization in schizonts, showing overlap of labeling with PfRON4. PF14_0578-HA labeling was peripheral in late stage schizonts (E), suggestive of an inner-membrane complex (IMC) or plasma membrane localization.Zuccala ES, Gout AM, Dekiwadia C, Marapana DS, Angrisano F, Turnbull L, Riglar DT, Rogers KL, Whitchurch CB, Ralph SA, Speed TP, Baum J. Subcompartmentalisation of Proteins in the Rhoptries Correlates with Ordered Events of Erythrocyte Invasion by the Blood Stage Malaria Parasite. PLoS One. 2012;7(9):e46160.
See original on MMP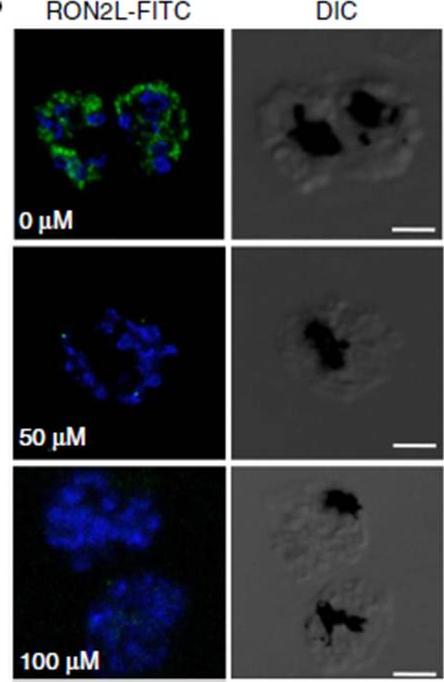
Immunofluorescence assay using FITC-labelled RON2L peptide. FITC-labelled RON2 peptide binds to AMA1 (blue)in the mature schizonts in the absence of inhibitors, whereas pre-incubation with inhibitor NCGC00015280 prevents binding of the peptide. the binding of RON2 to AMA1 triggers junction formation, which commits the merozoite for invasion. Although FITC-labelledPfRON2L binds to schizonts, binding is prevented in the presence of the inhibitor.Srinivasan P, Yasgar A, Luci DK, Beatty WL, Hu X, Andersen J, Narum DL, Moch JK, Sun H, Haynes JD, Maloney DJ, Jadhav A, Simeonov A, Miller LH. Disrupting malaria parasite AMA1-RON2 interaction with a small molecule prevents erythrocyte invasion. Nat Commun. 2013 4:2261.
See original on MMP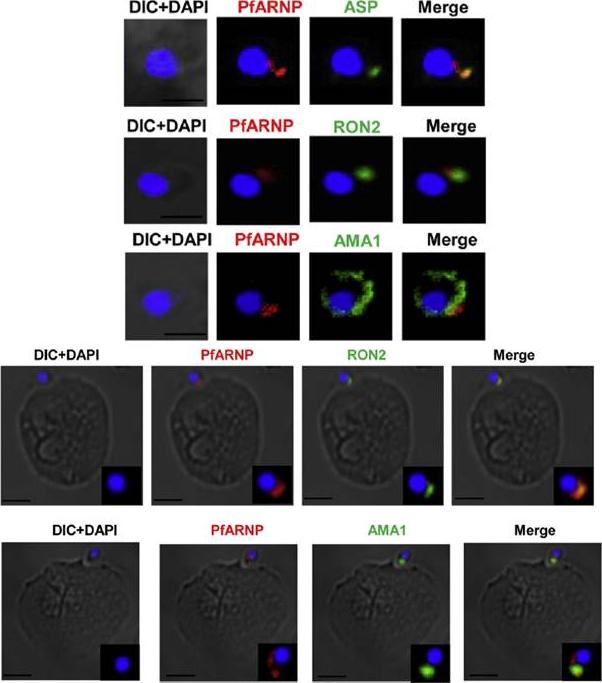
Localization of PfARNP in free merozites and at the tight junction. (Upper panel) IFA of merozoite using anti PfARNP antibody. Co-staining of PfARNP (red) was performed with ASP, RON2 (1973–2067 aa) and AMA1 (green) in free merozoites. Staining was observed at the apex of merozoite distinct from nuclear stain DAPI (blue). The staining merged perfectly well with ASP, RON2 and not with AMA1 confirming the localization of PfARNP in neck of rhoptries of merozoites. Scale bar shows 1 mm. (Lower panel) Localization of PfARNP during invasion of merozoite. IFA of PfARNP was performed with RON2 and AMA1, known markers of tight junction in Cytochalasin D treated merozoites invading to erythrocytes. The labeling of PfARNP (red) was observed at the site of attachment of merozoite to erythrocyte. The staining of PfARNP merged perfectly with RON2 and AMA1(green), indicating that PfARNP is present at the site of tight junction. The inset of each image show the optically zoomed view of invading merozoite. Scale bar shows 2 mm. Hans N, Singh S, Jain SK, Chauhan VS. Identification of novel rhoptry neck protein of Plasmodium falciparum. Mol Biochem Parasitol. 2013 188(1):34-9 Copyright Elsevier
See original on MMP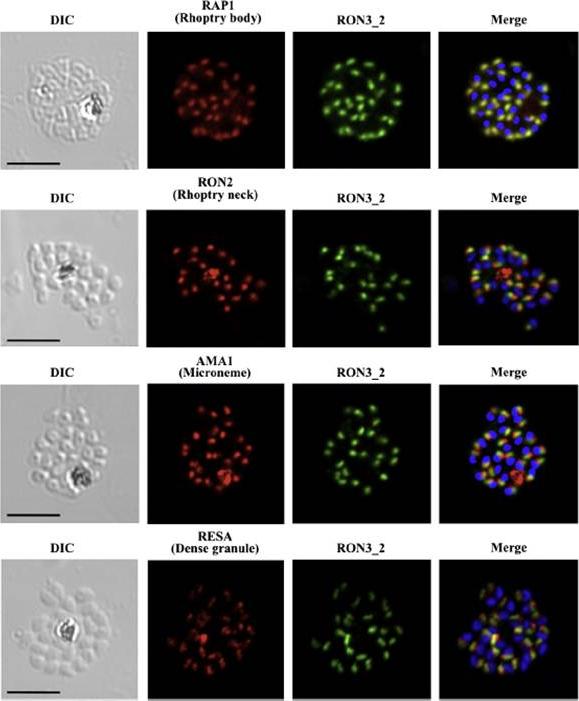
PfRON3 is expressed at the apical end of Plasmodium merozoites. Schizont and merozoite stage parasites were dual-labeled with antisera against PfRON3_2 and either PfRAP1 (rhoptry body marker), or PfRON2 (rhoptry neck marker), or PfAMA1 (microneme marker), or PfRESA (dense granule marker). Nuclei are visualized with DAPI in merged images shown in the right panels. Bars represent 5 μm. PfRON3 is localized to the but in the rhoptry body.Ito D, Han ET, Takeo S, Thongkukiatkul A, Otsuki H, Torii M, Tsuboi T. Plasmodial ortholog of Toxoplasma gondii rhoptry neck protein 3 is localized to the rhoptry body. Parasitol Int. 2011 60(2):132-8.
See original on MMP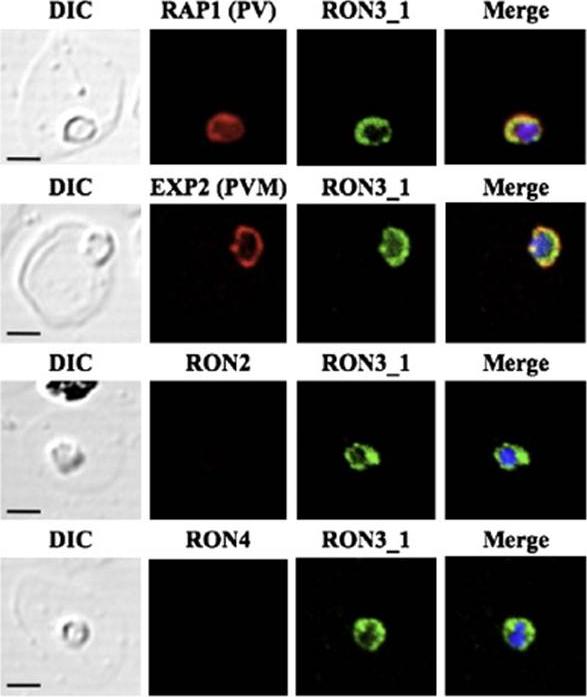
PfRON3 exists in parasitophorous vacuole in ring stage parasites. (A) Ring stage parasites were dual-labeled with antisera against PfRON3_1 and either PfRAP1 (PV marker), PfEXP2 (PVM marker), PfRON2, or PfRON4. Nuclei are visualized with DAPI in merged images shown in the right panels. Bars represent 2.5 μm.Ito D, Han ET, Takeo S, Thongkukiatkul A, Otsuki H, Torii M, Tsuboi T. Plasmodial ortholog of Toxoplasma gondii rhoptry neck protein 3 is localized to the rhoptry body. Parasitol Int. 2011 60(2):132-8.
See original on MMPMore information
| PlasmoDB | PF3D7_1452000 |
| GeneDB | PF3D7_1452000 |
| Malaria Metabolic Pathways | Localisation images Pathways mapped to |
| Previous ID(s) | PF14_0495 |
| Orthologs | PBANKA_1315700 , PCHAS_1319000 , PKNH_1230100 , PVP01_1255000 , PVX_117880 , PY17X_1319500 |
| Google Scholar | Search for all mentions of this gene |