PF3D7_1370300 membrane associated histidine-rich protein (MAHRP1)
Disruptability [+]
| Species | Disruptability | Reference | Submitter |
|---|---|---|---|
| P. falciparum 3D7 |
Possible |
USF piggyBac screen (Insert. mut.) | USF PiggyBac Screen |
| P. falciparum 3D7 |
Possible |
18410498 \"We disrupted the gene for the membrane-associated histidine-rich protein 1 (MAHRP1). MAHRP1 is not essential for parasite viability or Maurer\'s cleft formation; however, in its absence, these organelles become disorganized in permeabilized cells. Maurer\'s cleft-resident proteins and transit cargo are exported normally in the absence of MAHRP1; however, the virulence determinant, PfEMP1, accumulates within the parasite, is depleted from the Maurer\'s clefts and is not presented at the red blood cell surface. Complementation of the mutant parasites with mahrp1 led to the reappearance of PfEMP1 on the infected red blood cell surface, and binding studies show that PfEMP1-mediated binding to CD36 is restored. These data suggest an important role of MAHRP1 in the translocation of PfEMP1 from the parasite to the host cell membrane.\" |
Theo Sanderson, Francis Crick Institute |
Mutant phenotypes [+]
| Species | Stage | Phenotype | Reference | Submitter |
|---|---|---|---|---|
| P. falciparum 3D7 | Asexual |
Difference from wild-type |
18410498 \"We disrupted the gene for the membrane-associated histidine-rich protein 1 (MAHRP1). MAHRP1 is not essential for parasite viability or Maurer\'s cleft formation; however, in its absence, these organelles become disorganized in permeabilized cells. Maurer\'s cleft-resident proteins and transit cargo are exported normally in the absence of MAHRP1; however, the virulence determinant, PfEMP1, accumulates within the parasite, is depleted from the Maurer\'s clefts and is not presented at the red blood cell surface. Complementation of the mutant parasites with mahrp1 led to the reappearance of PfEMP1 on the infected red blood cell surface, and binding studies show that PfEMP1-mediated binding to CD36 is restored. These data suggest an important role of MAHRP1 in the translocation of PfEMP1 from the parasite to the host cell membrane.\" |
Theo Sanderson, Francis Crick Institute |
Imaging data (from Malaria Metabolic Pathways)
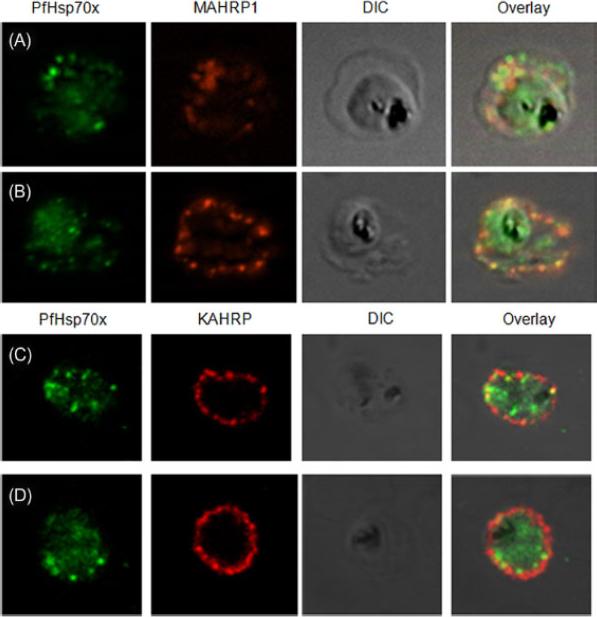
PfHsp70-x partially associates with Maurer’s clefts but not with knobs: (A–B) Panel I: the signal for PfHsp70-x (green) was obtained in the parasite compartment along with few punctate spots in the erythrocyte compartment. Panel II: MAHRP1 (red) stained discrete foci representative of Maurer’s clefts in the erythrocyte periphery. Panel III: the DIC image of the infected erythrocyte. Panel IV: merged image overlaid with DIC image reveals that MAHRP1 and PfHsp70-x partially co-localize in the erythrocyte compartment, suggesting that PfHsp70-x possibly associates with Maurer’s clefts. (C-D) Panel I: the signal for PfHsp70-x (green) was obtained in the parasite compartment along with few punctate spots in the erythrocyte compartment. Panel II: KAHRP (red), being a constituent of knobs, stained the entire erythrocyte membrane. Panel III shows the DIC image of the infected erythrocyte. Panel IV: no co-localization is observed between KAHRP and PfHsp70-x, suggesting that PfHsp70-x does not associate with knobs on the infected erythrocyte membrane.Grover M, Chaubey S, Ranade S, Tatu U. Identification of an exported heat shock protein 70 in Plasmodium falciparum. Parasite. 2013;20:2.
See original on MMP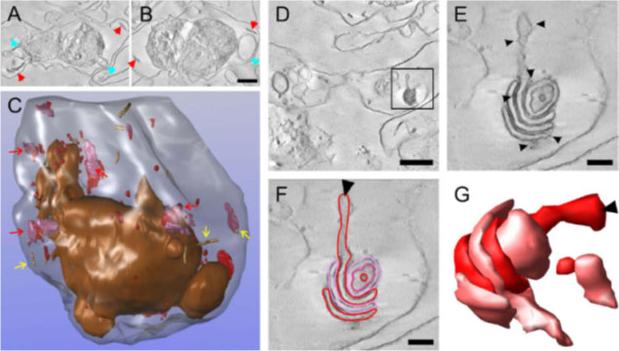
Three-dimensional (3-D) reconstruction of an entire Plasmodium falciparum strain D10-infected red blood cell (RBC) from electron tomograms of serial sections. Purified D10 parasite-infected RBCs were permeabilized with Equinatoxin II (Eqt II) and labelled with anti-membrane-associated histidine-rich protein-1 (MAHRP1), and immunogold protein A, fixed, stained, embedded in resin and sectioned (300 nm). Twenty-five serial sections were collected through a late trophozoite-infected RBC. (A and B) Selected virtual sections (12 nm) with Maurer’s clefts and tubulovesicular network (TVN) indicated with red and blue arrowheads. Scale bar, 1 lm. (C) Individual structures were segmented. The RBC membrane is rendered in translucent grey and the parasitophorous vacuole (PV) membrane and extensions from the PV membrane in bronze. Maurer’s cleft lamellae are depicted in red and mauve and are sometimes present as individual lamellae but often present as stacks. (Some examples are indicated with red arrows). Tubular structures are shown as beige cylinders (yellow arrows). (D) Virtual section (within the 15th physical section) with an individual Maurer’s cleft stack marked with a rectangle. Scale bar, 1 lm. (E) Virtual section of the same Maurer’s cleft stack from tomogram data collected at higher magnification. The presence of the gold particles (arrowheads) indicates labelling of the Maurer’s cleft resident, MAHRP1. This Maurer’s cleft stack spanned three sections. (F and G) Different lamellae of the Maurer’s clefts are indicated in red and pink in a virtual section and a rendered image. The arrowheads indicate the same extended region of the Maurer’s cleft in C and D. Scale bars, 200 nm.Hanssen E, Carlton P, Deed S, Klonis N, Sedat J, Derisi J, Tilley L. Whole cell imaging reveals novel modular features of the exomembrane system of the malaria parasite, Plasmodium falciparum. Int J Parasitol. 2010 Jan;40(1):123-134. Copyright Elsevier 2010.
See original on MMP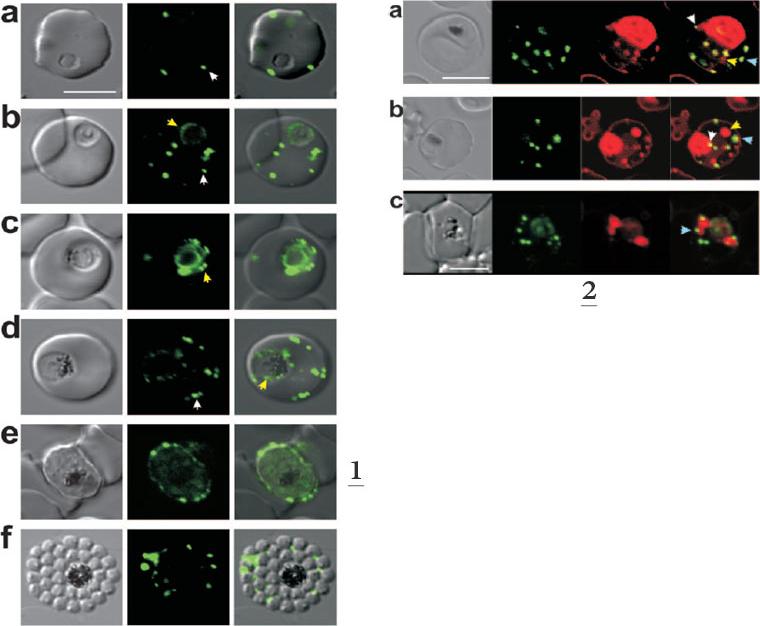
1. Expression of MAHRP11-249-GFP at different stages of the intraerythrocytic cycle of P. falciparum. The images represent a DIC image, the GFP fluorescence signal, and an overlay of these images. Ring and trophozoite stage parasites (a to d) show puncta of fluorescence in the RBC cytoplasm which appear to represent peripheral Maurer’s clefts (white arrows). Some cells (c) show a ring of “beads” of fluorescence around the PV. These foci may represent nascent Maurer’s clefts (yellow arrows). Mature schizont-stage parasites (e) show flattening of the peripheral Maurer’s clefts against the RBC membrane.2. Dual labeling of MAHRP11-249-GFP ransfectants with BODIPY-ceramide. The images represent (from left to right) a DIC image, GFP fluorescence, BODIPY-ceramide fluorescence, and an overlay of the GFP (green) and BODIPY-ceramide (red) images. The parasite membranes are intensely labeled with the lipid probe. Some extensions of the PV membrane are dotted with foci of MAHRP1-GFP (white arrows). Some of the BODIPY-labeled structures (probably TVN extensions and buds) are not labeled with GFP (yellow arrows), while others (presumably Maurer’s clefts) are labeled with GFP (blue arrows). Bar, 5 mm.Spycher C, Rug M, Klonis N, Ferguson DJ, Cowman AF, Beck HP, Tilley L. Genesis of and trafficking to the Maurer's clefts of Plasmodium falciparum-infected erythrocytes. Mol Cell Biol. 2006 26:4074-85.
See original on MMP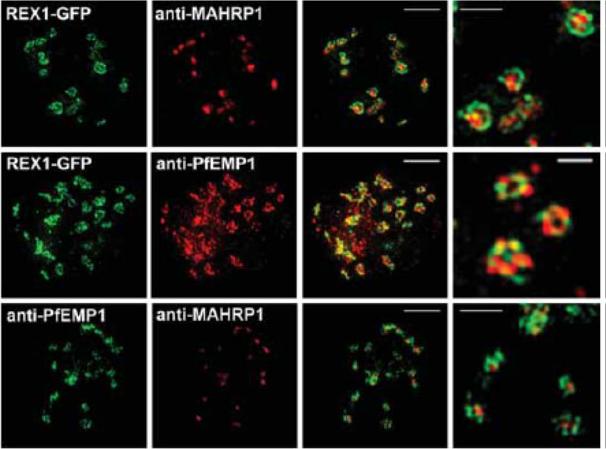
3D-SIM analysis of the organization of Maurer's cleft resident proteins. REX1-GFP transfectants were permeabilized with EqtII and labeled with antibodies recognizing GFP, PfEMP1 (ATS) or MAHRP1 and 3D stacks were generated by 3D-SIM. Higher magnification images at right. Scale bars = 2 μm, first 3 columns; 500 nm, last column.McMillan PJ, Millet C, Batinovic S, Maiorca M, Hanssen E, Kenny S, Muhle RA, Melcher M, Fidock DA, Smith JD, Dixon MW, Tilley L. Spatial and temporal mapping of the PfEMP1 export pathway in Plasmodium falciparum. Cell Microbiol. 2013 15(8):1401-18
See original on MMP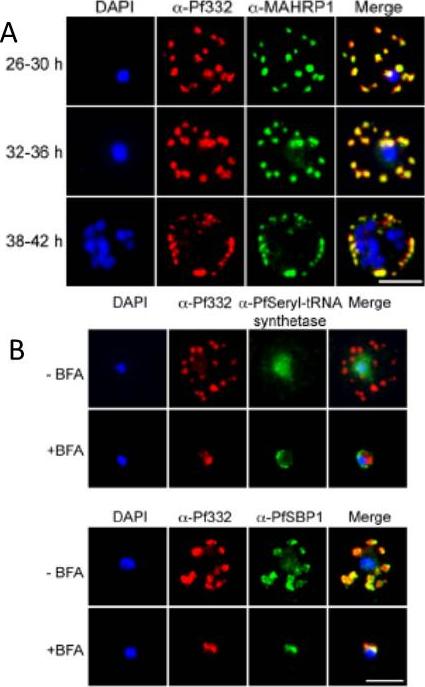
A. IFA of air-dried monolayers of pRBC collected at each of the three indicated time-points. Slides were probed with mouse monoclonal anti-Pf332-DBL (red) and rabbit polyclonal anti-PfMAHRP1 antibodies (green). The parasite was counterstained with DAPI (blue). Scale bar indicates 5 mm. Pf332 displayed a close association with Maurer’s clefts throughout trophozoite maturation and schizogonyB. Air-dried monolayers of BFA+ and BFA2 pRBC were probed with monoclonal anti-Pf332-DBL (red) and polyclonal anti-PfSeryl-tRNA synthetase antibodies (green), or polyclonal anti-Pf332-E200 (red) and polyclonal anti-PfSBP1 antibodies (green). The parasite was counterstained with DAPI (blue). Scale bar indicates 5 mm. Synchronous early ring-stage pRBC were treated with (+) or without BFA for 20 h and inhibition of protein export was verified by IFA. As controls, we included antibodies towards the BFA sensitive SBP1, and the nonexported Seryl-tRNA synthetase. In the absence of BFA, both the anti-Pf332 and anti-SBP1 antibodies stained Maurer’s clefts, whereas the Seryl-tRNA synthetase antibodies only stained the parasiteNilsson S, Angeletti D, Wahlgren M, Chen Q, Moll K. Plasmodium falciparum Antigen 332 Is a Resident Peripheral Membrane Protein of Maurer's Clefts. PLoS One. 2012;7(11):e46980.
See original on MMP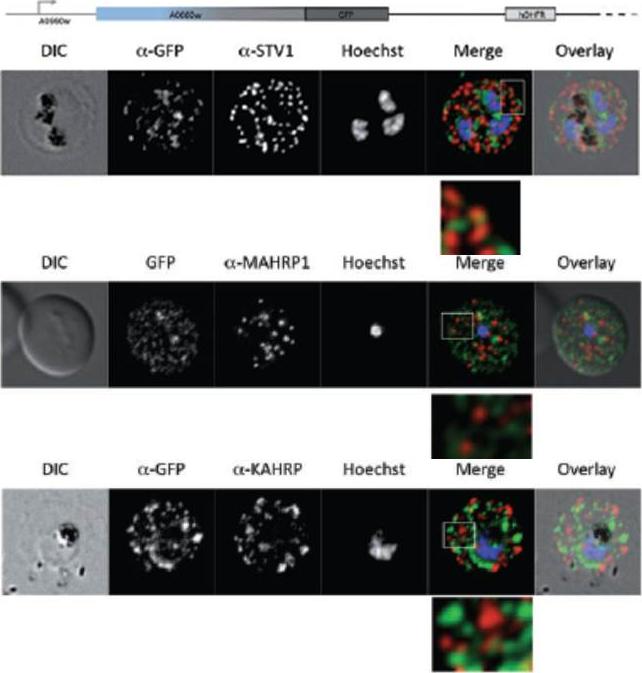
Parasites were tagged by single cross-over homologous recombination, the endogenous gene locus to include the GFP coding sequence. PFA660INT. Co-immunofluorescence analysis on erythrocytes infected with PFA660INT, using anti-sera directed against STEVOR (A), MAHRP1 MAL13P1.413 (B), KAHRP PFB0100c (C). Fluorescence channels are shown individually in black/white for highest contrast. All images are maximal projections of Z-stack serial sections. In merge image green, GFP; red, STEVOR (A), MAHRP (B), KAHRP (C) or ATS domain of PfEMP1 (D); blue, Hoechst. Inset shows enlargement of merge (white box). The fusion protein labelled punctate structures within the infected erythrocytebut clearly not in Maurer’s clefts.Külzer S, Rug M, Brinkmann K, Cannon P, Cowman A, Lingelbach K, Blatch GL, Maier AG, Przyborski JM. Parasite encoded Hsp40 proteins define novel mobile structures in the cytosol of the P. falciparum infected erythrocyte. Cell Microbiol. 2010 12(10):1398-420. Copyright John Wiley & Sons Ltd. 2010.
See original on MMP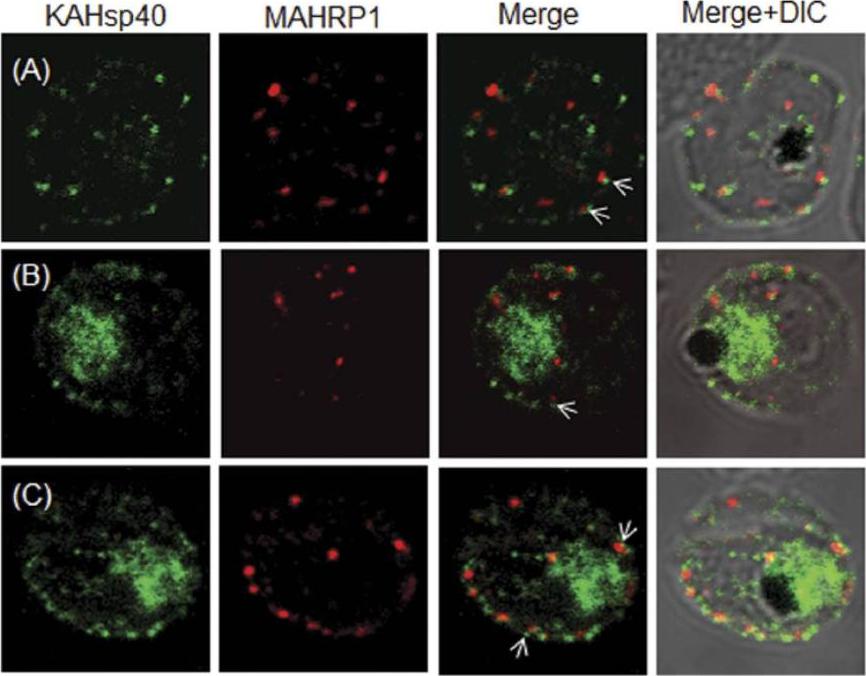
KAHsp40 does not associate with Maurer’s cleft. (A–C) IFA reveals that both KAHsp40 and MAHRP1 are present in discrete and different foci in the infected erythrocyte, however, they do not co-localize with each other in spite of signals being in close proximity (highlighted by white arrows). The images shown have been taken at the trophozoite stage.Acharya P, Chaubey S, Grover M, Tatu U. An Exported Heat Shock Protein 40 Associates with Pathogenesis-Related Knobs in Plasmodium falciparum Infected Erythrocytes. PLoS One. 2012;7(9):e44605.
See original on MMP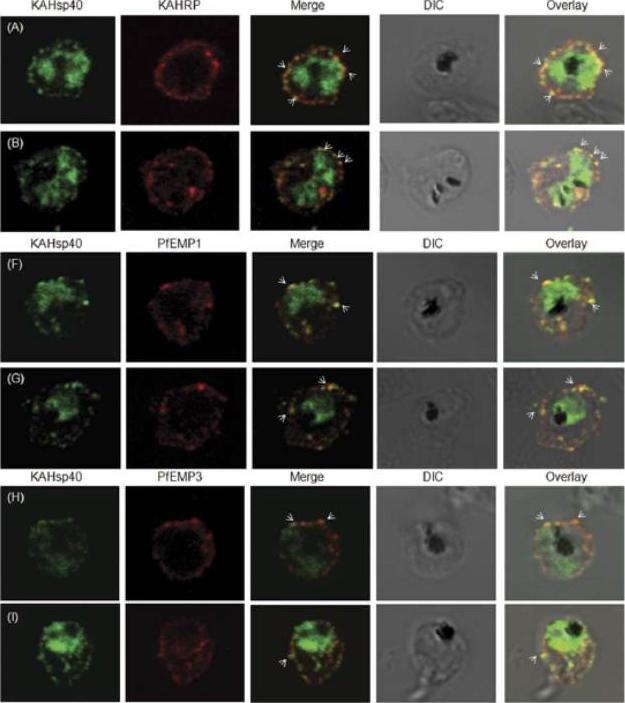
KAHsp40 associates with knobs on the infected erythrocyte membrane. (A-B) IFA reveals that KAHsp40 and KAHRP co-localize in the erythrocyte periphery near the erythrocyte plasma membrane. White arrows indicate the discrete foci in which they co-localize. KAHsp40 and PfEMP1 and KAHsp40 and PfEMP3 co-localize with each other on the erythrocyte plasma membrane indicating the close association of KAHsp40 with knobs.Acharya P, Chaubey S, Grover M, Tatu U. An Exported Heat Shock Protein 40 Associates with Pathogenesis-Related Knobs in Plasmodium falciparum Infected Erythrocytes. PLoS One. 2012;7(9):e44605.
See original on MMP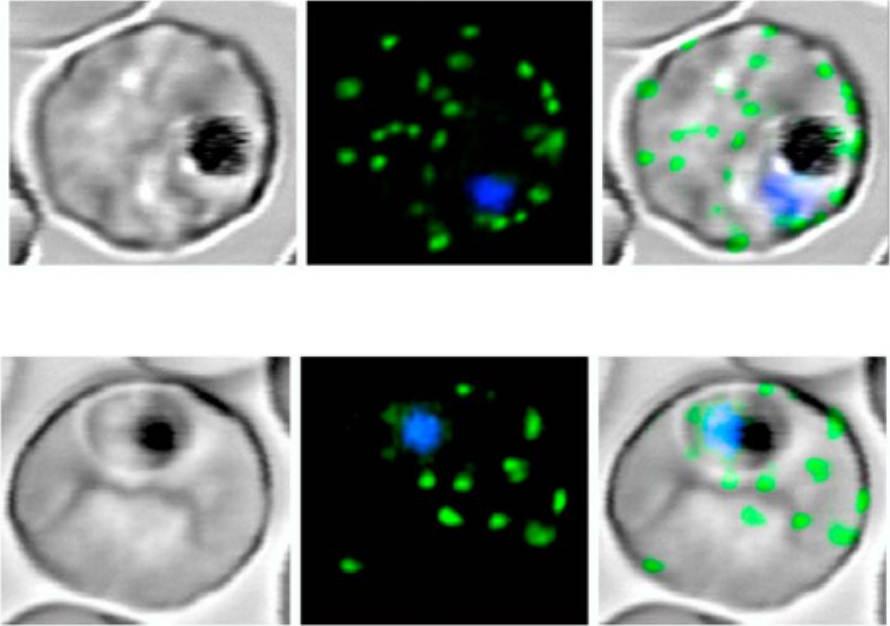
Live P. falciparum-infected erythrocytes were incubated in physiological Ringer’s solution and viewed, at 37 oC, using an LSM510 confocal laser scanning microscope. Confocal images of live P. falciparum-infected erythrocytes expressing SBP. Left images, differential interference contrast (DIC); middle images, GFP fluorescence and nuclear staining with Hoechst; right images, overlay. Scale bar, 4 μm.The right column shows confocal live cell images of P. falciparum-infected erythrocytes expressing MAHRP. Both proteins localize to the Maurer’s clefts.Saridaki T, Fröhlich KS, Breton CB, Lanzer M. Export of PfSBP1 to the Plasmodium falciparum Maurer's clefts. Traffic. 2008 10(2):137-52.
See original on MMP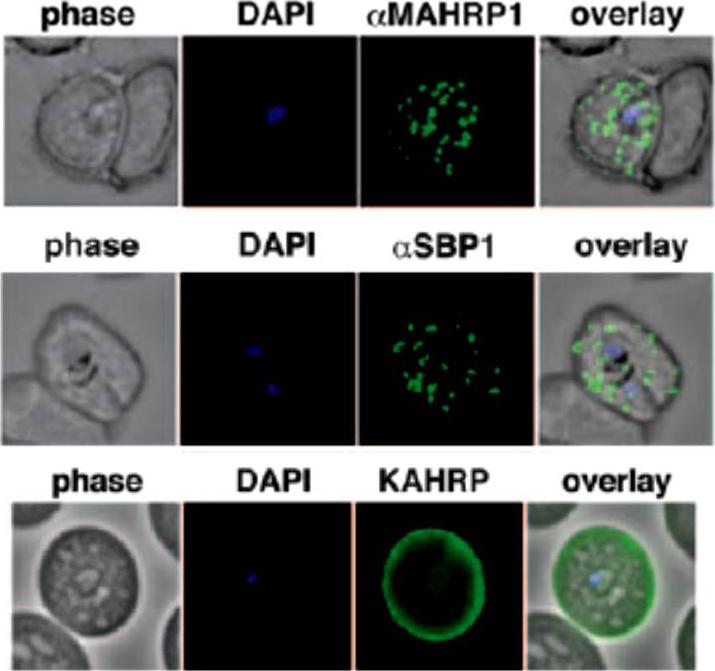
Localization of the resident Maurer clefts markers PfSBP1 and PfMAHRP1 in CS2. Both proteins trafficked to Maurer’s clefts. KAHRP is trafficked normally to the erythrocyte membrane and assembled into knob structures.Maier AG, Rug M, O'Neill MT, Beeson JG, Marti M, Reeder J, Cowman AF. Skeleton-binding protein 1 functions at the parasitophorous vacuole membrane to traffic PfEMP1 to the Plasmodium falciparum-infected erythrocyte surface. Blood. 2007 109:1289-97.
See original on MMP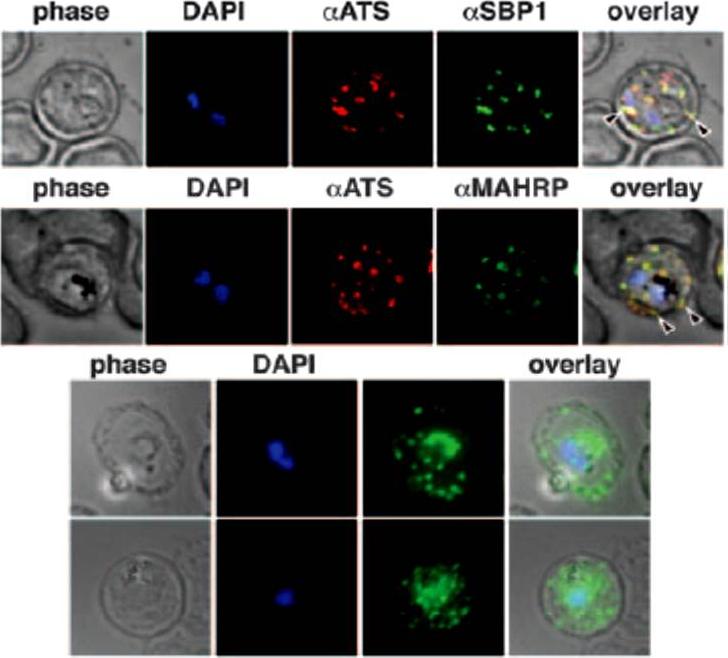
Immunofluorescence study to assess trafficking of PfEMP1 in erythrocytes infected with CS2 parasites. A. Cells were probed with anti-ATS (anti-PfEMP1) and anti-SBP1 antibodies showed typical PfEMP1 localization in Maurer clefts in the erythrocyte, B. Colocalization with anti-ATS and anti-MAHRP antibodies to Maurer clefts. C. Expression of PfEMP1-YFP in CS2-infected erythrocytes are shown by anti-GFP labeling. The fluorescence is localized in the parasite, but the majority of the protein is transported into the erythrocyte cytosol and shows a characteristic Maurer clefts staining.Maier AG, Rug M, O'Neill MT, Beeson JG, Marti M, Reeder J, Cowman AF. Skeleton-binding protein 1 functions at the parasitophorous vacuole membrane to traffic PfEMP1 to the Plasmodium falciparum-infected erythrocyte surface. Blood. 2007 109:1289-97. >
See original on MMP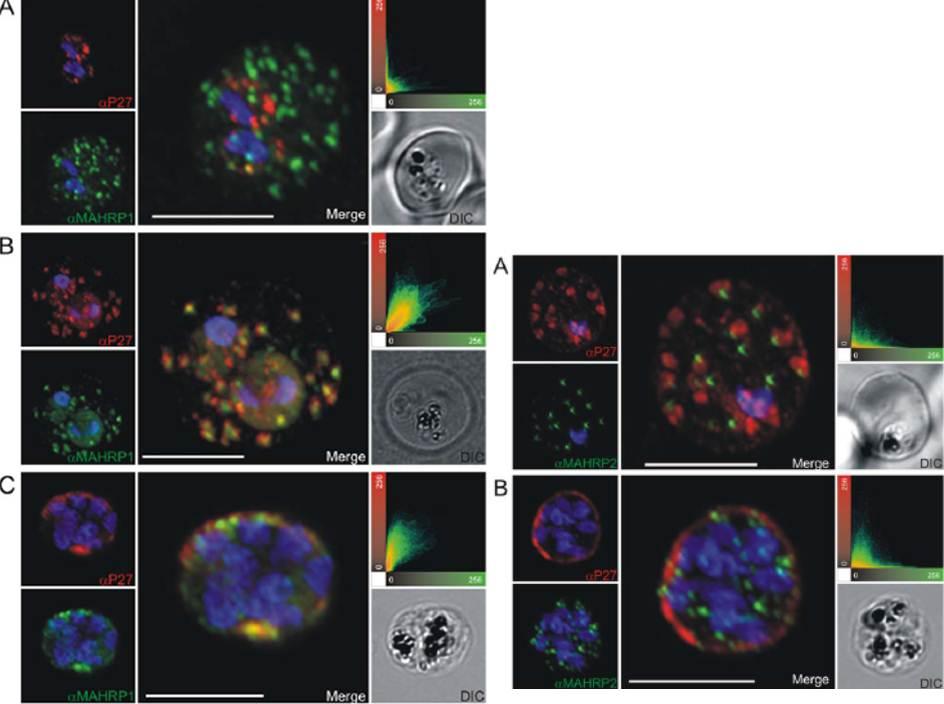
Left: Co-localization of Tex1 with MAHRP1. P27-specific polyclonal rabbit sera was used to detect Tex1 (red). Co-localization was performed using MAHRP1 polyclonal mouse sera (green). Co-localization was performed in ring stage (A) trophozoite (B) and schizont stage (C) infected RBC. Nuclear DNA was stained with DAPI (blue), Transmission image (DIC), Scale bar: 5 mm.Right: Tex1 localization in trophozoite and schizont stages with respect to newly described structures called tethers. Colocalization of Tex1 (red) with MAHRP2 (green) A) in trophozoite stages and B) in schizont stages. Nuclear DNA was stained with DAPI (blue), Transmission image (DIC), Scale bar: 5 mm.Kulangara C, Luedin S, Dietz O, Rusch S, Frank G, Mueller D, Moser M, Kajava AV, Corradin G, Beck HP, Felger I. Cell biological characterization of the malaria vaccine candidate trophozoite exported protein 1. PLoS One. 2012;7(10):e46112.
See original on MMP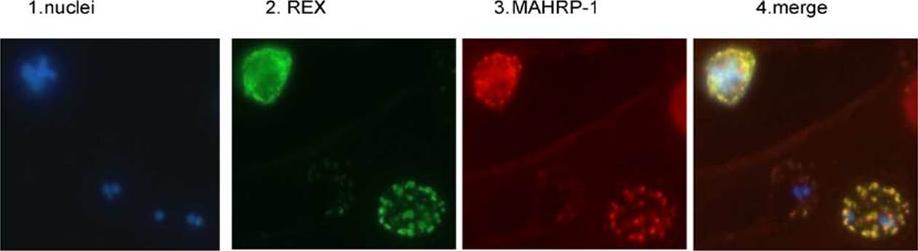
REX is located in the Maurer’s clefts. Panel 1 depicts nuclear staining (blue) of a field of 3D7 parasites. Panel 2 depicts the same field stained with REX antiserum (green). Panel 3 is the same field stained with MAHRP-1 antiserum (red). Panel 4 is a merge of the first three panels.Hawthorne PL, Trenholme KR, Skinner-Adams TS, Spielmann T, Fischer K, Dixon MW, Ortega MR, Anderson KL, Kemp DJ, Gardiner DL. A novel Plasmodium falciparum ring stage protein, REX, is located in Maurer's clefts. Mol Biochem Parasitol. 2004 136:181-9. Copyright Elsevier 2009. PMID:
See original on MMP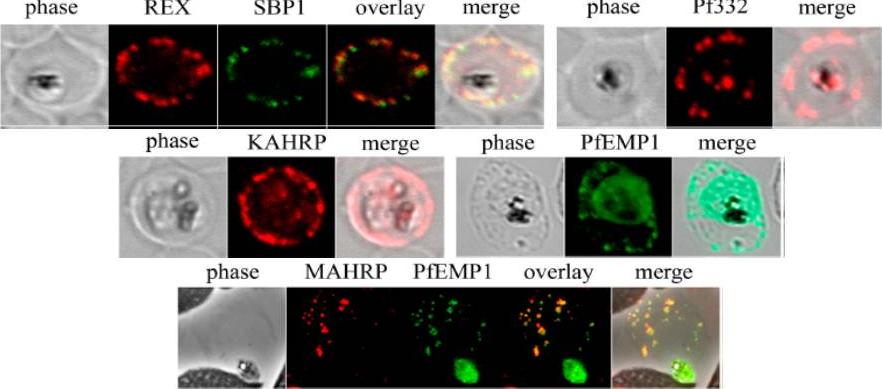
Analysis of expression and localization of Maurer’s cleft–associated proteins in IRBCs. Confocal immunofluorescence analysis of resident (REX-PFI1735c, SBP1-PFE0065w, and Pf332-PF11_0507) or transiently associated (KAHRP-PFB0100c and PfEMP1) Maurer’s cleft proteins. Colocalization of the resident Maurer’s cleft marker MAHRP-MAL13P1.413 with PfEMP1 in streptolysin O–pretreated IRBCs demonstrates that PfEMP1 is associated with Maurer’s clefts.Cooke BM, Buckingham DW, Glenister FK, Fernandez KM, Bannister LH, Marti M, Mohandas N, Coppel RL. A Maurer's cleft-associated protein is essential for expression of the major malaria virulence antigen on the surface of infected red blood cells. J Cell Biol. 2006 172:899-908.
See original on MMP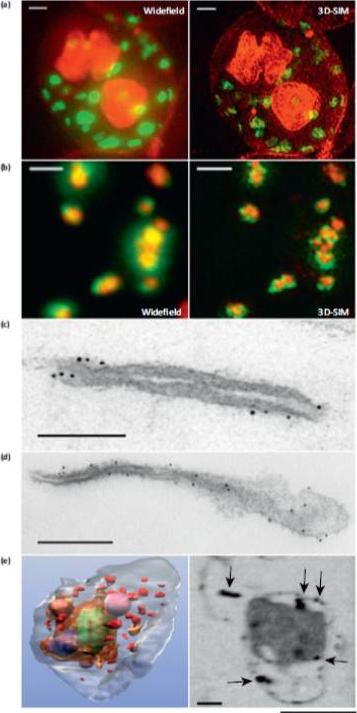
Microscopy methods for localizing Plasmodium falciparum Maurer’s cleft-associated proteins. (a) Plasmodium falciparum transfectants expressing REX1-GFP (green) costained with a membrane stain (red) and imaged using conventional widefield fluorescence microscopy and 3D structured illumination microscopy (3D-SIM). Scale bars = 1 mm. (b) Immunofluores-cence microscopy of P. falciparum REX1-GFP transfectants labeled with anti-GFP (green) and anti-MAHRP1 (red) antibodies. The sample was imaged using conventional widefield fluorescence microscopy and 3D-SIM. Scale bars = 1 mm. (c, d) Immuno-electron microscopy. Red blood cells (RBCs) infected with trophozoite stage REX1-GFP transfectants [(c) scale bar = 100 nm)] or MAHRP1-GFP trans-fectants [(d) (scale bar = 200 nm)] were permeabilized with Equinatoxin II and labeled with anti-GFP antiserum followed by 6 nm gold-labeled protein A. (e) Left hand panel: rendered cryo-X-ray tomogram of an Equinatoxin II permeabilized trophozoite-infected RBC. The surface of the trophozoite is rendered in brown, the nucleus in green, the digestive vacuole in purple. Hemoglobin containing vesicles in the RBC cytoplasm are rendered in magenta and Maurer’s clefts in red. Right hand panel: cryo-X-ray micrograph of an infected RBC. MAHRP1-GFP transfectant-infected RBCs were permeabilized and labeled with mouse anti-GFP antiserum followed by 6 nm gold-labeled protein A. The size of the gold particles was increased by silver enhancement. Dark deposits in the RBC cytoplasm (arrowheads) reveal labeling of the Maurer’s clefts. Scale bars = 500 nm.Woodcroft BJ, McMillan PJ, Dekiwadia C, Tilley L, Ralph SA. Determination of protein subcellular localization in apicomplexan parasites. Trends Parasitol. 2012 Dec;28(12):546-54.
See original on MMP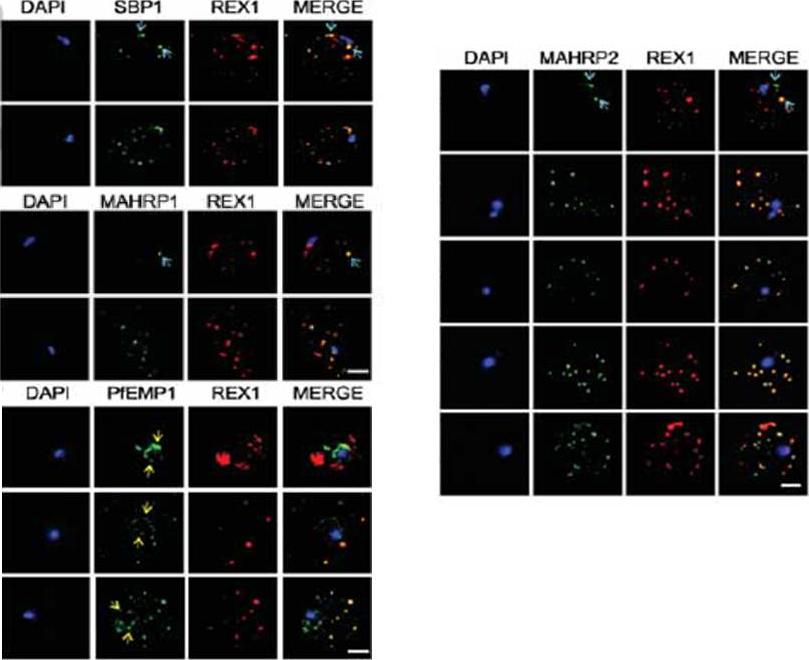
Immunofluorescence microscopy showing staggered delivery of different exomembrane components. (A-D) Infected RBCs were synchronized to a 1 h window, samples were collected at 2 h intervals and smears were fixed with acetone and stained with antibodies recognizing REX1, MAHRP1, SBP1, MAHRP2 and PfEMP1 (ATS) and co-stained with DAPI. Images are presented at time points before and after delivery to the RBC cytoplasm. Scale bars = 3 μm. McMillan PJ, Millet C, Batinovic S, Maiorca M, Hanssen E, Kenny S, Muhle RA, Melcher M, Fidock DA, Smith JD, Dixon MW, Tilley L. Spatial and temporal mapping of the PfEMP1 export pathway in Plasmodium falciparum. Cell Microbiol. 2013 15(8):1401-18
See original on MMP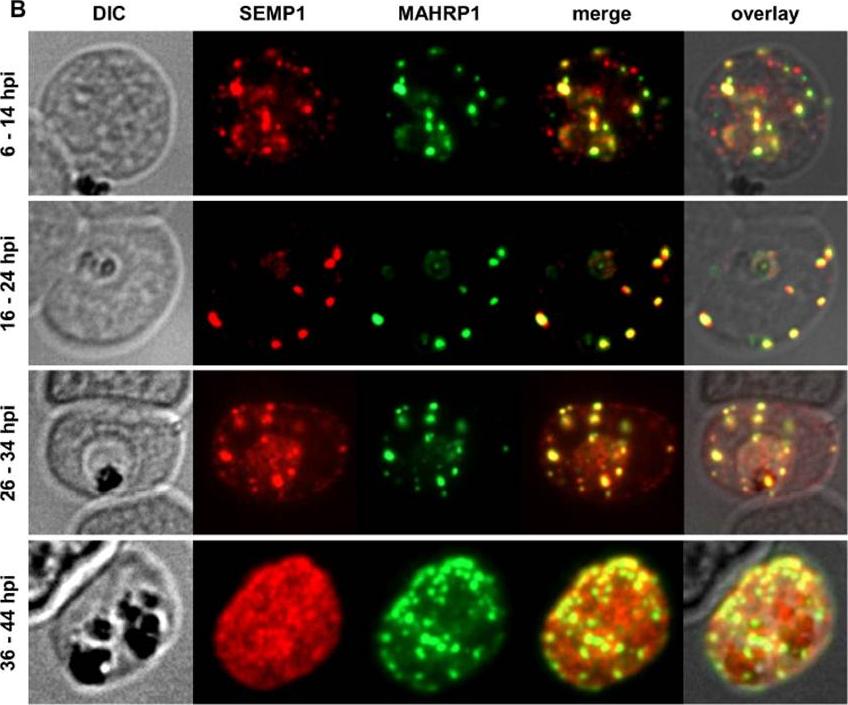
Localization and expression of endogenous SEMP1 in 3D7 wild-type parasites. IFAs of MeOH-fixed RBCs infected with 3D7 wild-type parasites synchronized at timepoints 6–14 hours post invasion (hpi), 16–24 hpi, 26–34 hpi and36–44 hpi. Cells were co-labelled with mouse a-SEMP1 and rabbit a-MAHRP1 serum. Dual-labelling IFAs of sorbitol-synchronized parasites at 6–14 hours post invasion (hpi), 16–24 hpi, 26–34 hpi, and 36–44 hpi showed that SEMP1, similarly to MAHRP1, is exported early after invasion to MCs where it partially co-localized with MAHRP1Dietz O, Rusch S, Brand F, Mundwiler-Pachlatko E, Gaida A, Voss T, Beck HP. Characterization of the Small Exported Plasmodium falciparum Membrane Protein SEMP1. PLoS One. 2014 9(7):e103272.
See original on MMP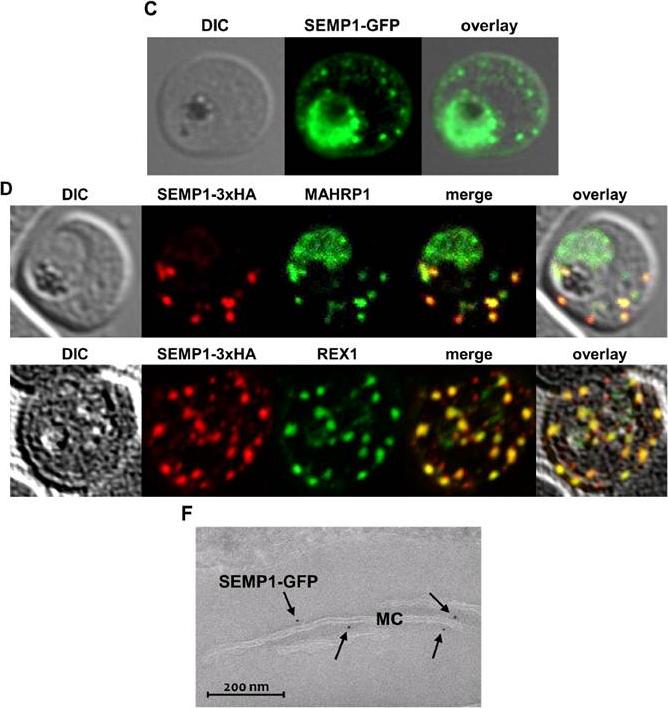
C: Live cell imaging of 3D7 parasites expressing SEMP1 with C-terminal tagged GFP. D: Immunofluorescence assays of MeOHfixed RBCs infected with 3D7 expressing SEMP1 with a C-terminal 3xHA tag, co-labelled with rat a-HA and either rabbit a-MAHRP1 or rabbit a-REX1 antibodies. D: Scatter plot of co-localization of SEMP1 and REX1 in SEMP1-3xHA parasites. E: Electron microscopy (EM) of RBCs infected with 3D7 expressing SEMP1 with a C-terminal GFP tag labelled with rabbit a-GFP antibodies and decorated with 5 nm gold conjugated Protein A. GFP-tagged protein was exported (c). SEMP1-3xHA co-localized with MAHRP1 and REX1 to the MCs. Electron microscopy using antibodies against GFP confirmed the localization of SEMP1-GFP at the MCs (F). Dietz O, Rusch S, Brand F, Mundwiler-Pachlatko E, Gaida A, Voss T, Beck HP. Characterization of the Small Exported Plasmodium falciparum Membrane Protein SEMP1. PLoS One. 2014 9(7):e103272.
See original on MMP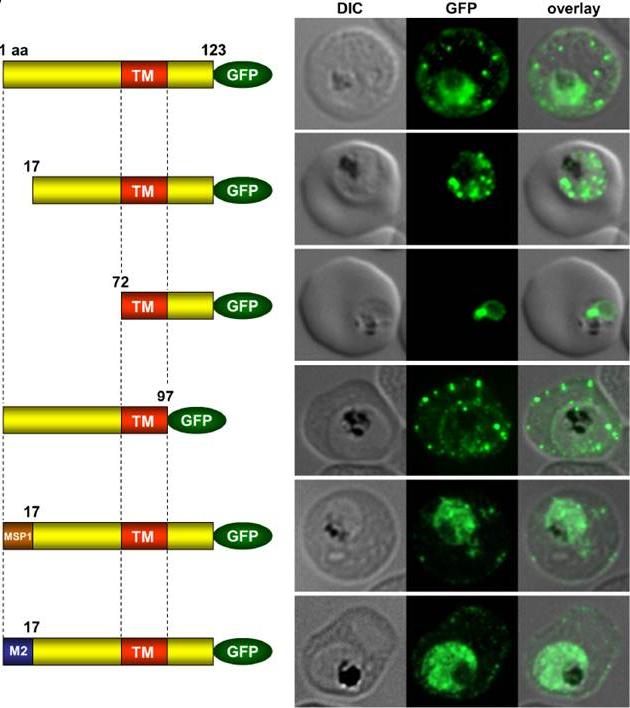
Immunofluorescence assays of MeOH-fixed RBCs infected with SEMP1-KO parasites expressing full-length and truncated or mutated forms of SEMP1 C-terminally fused to GFP. Expressed SEMP1 was labelled with rabbit a-GFP antibodies. The transmembrane domain is depicted in red (TM), a MSP1 signal peptide in brown (MSP1) and the MAHRP2 N-terminus in blue (M2). deletion of the complete C-terminus of SEMP1 (SEMP11–97-GFP) did not impair export and resulted in correct localisation whilst deletion of the first 16 amino acids (SEMP117–123-GFP) of the N-terminus resulted in an impaired export from the parasite. The deletion of 71 amino acids (SEMP172–123-GFP) of the N-terminus prevented export and seemed to have resulted in concentration of SEMP1 in or at the endoplasmic reticulum. If the first 16 amino acids of SEMP1 were replaced by the corresponding amino acids of MAHRP2 (M21–16SEMP117–123-GFP) or the classical signal peptide of MSP1 (MSP11–16SEMP117–123-GFP) the fusion protein was exported in both cases albeit with lower efficiency.Dietz O, Rusch S, Brand F, Mundwiler-Pachlatko E, Gaida A, Voss T, Beck HP. Characterization of the Small Exported Plasmodium falciparum Membrane Protein SEMP1. PLoS One. 2014 9(7):e103272.
See original on MMP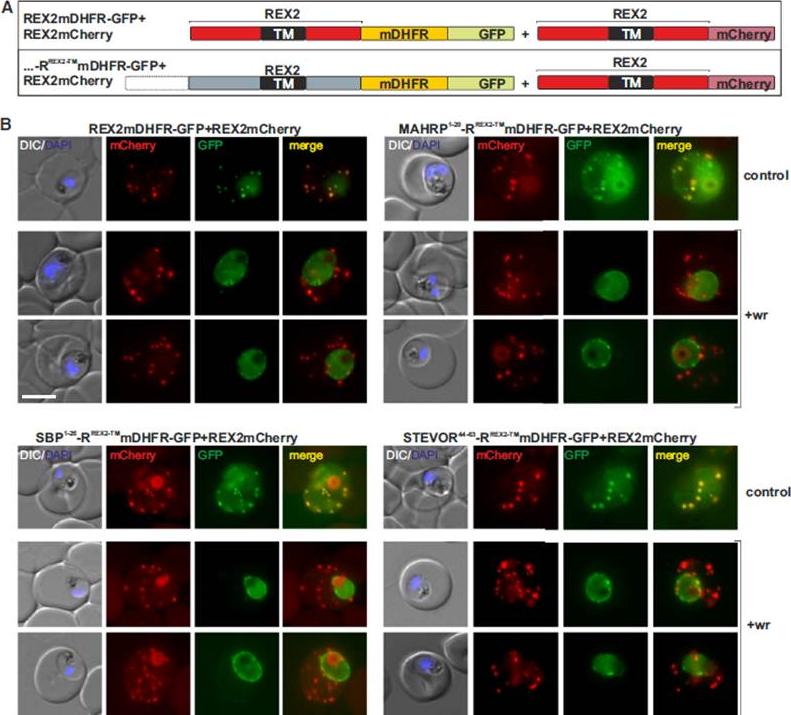
Blocking Unfolding Arrests Export of PNEPs. (A) Schematic of the constructs of the cell lines shown in (B). (B) Parasites expressing REX2mCherry (red) together with the mDHFR-GFP-tagged constructs (green) indicated above each panel. Two images are shown per cell line in the WR99210 (+wr)-treated samples to demonstrate that in different cells the parasite peripheral staining of the blocked protein displayed either a smooth or a more focal pattern. Merge, overlay of the red and green signals. The size bar represents 5 mm.Grüring C, Heiber A, Kruse F, Flemming S, Franci G, Colombo SF, Fasana E, Schoeler H, Borgese N, Stunnenberg HG, Przyborski JM, Gilberger TW, Spielmann T. Uncovering common principles in protein export of malaria parasites. Cell Host Microbe. 2012 12(5):717-29.
See original on MMP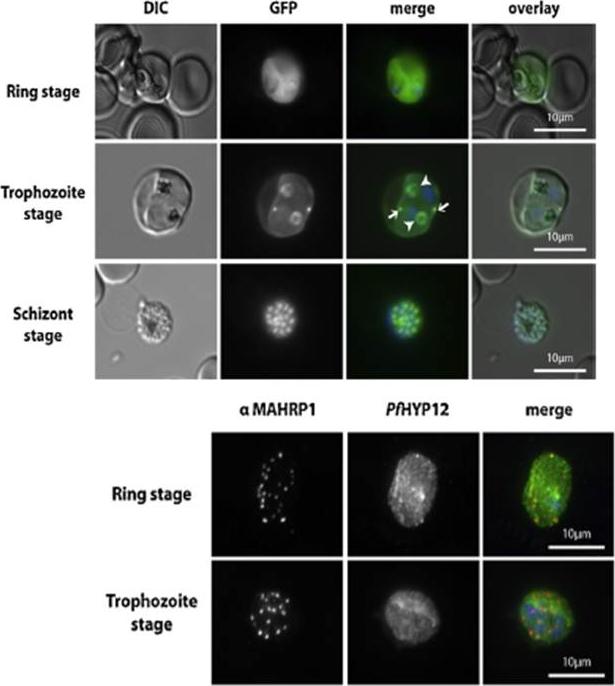
PfHYP12 is exported to the RBC cytoplasm and localizes to distinct intra-parasitic structures. Upper panel: Epifluorescence microscopy of erythrocytes infected with PfHYP12-GFP expressing 3D7 parasites. Images of live cells infected with parasites at three distinct stages are displayed. Merge image: Green, GFP; blue, Hoechst. DIC. Lower panel: Localization of PfHYP12-GFP by IFA. Cells were prepared for microscopy from asynchronous blood stage cultures. Representative co-immunofluorescence pictures are shown for two different stages of infection. Acetone/methanol (90%/10%) fixed smears were stained with mouse a-MAHRP1 (1:1000) (center left) and chicken a-GFP (1:500, Abcam) antibodies (center right) followedby incubation with Alexa Fluor-conjugated secondary antibodies in order to visualize PfHYP12-GFP and Maurer’s clefts, respectively. Merge image: red, MAHRP1; green, GFP; blue, Hoechst. Scale bars: 10 mm. Petersen W, Matuschewski K, Ingmundson A. Trafficking of the signature protein of intra-erythrocytic Plasmodium berghei-induced structures, IBIS1, to P. falciparum Maurer's clefts. Mol Biochem Parasitol. 2015 200(1-2):25-29. PMID:
See original on MMP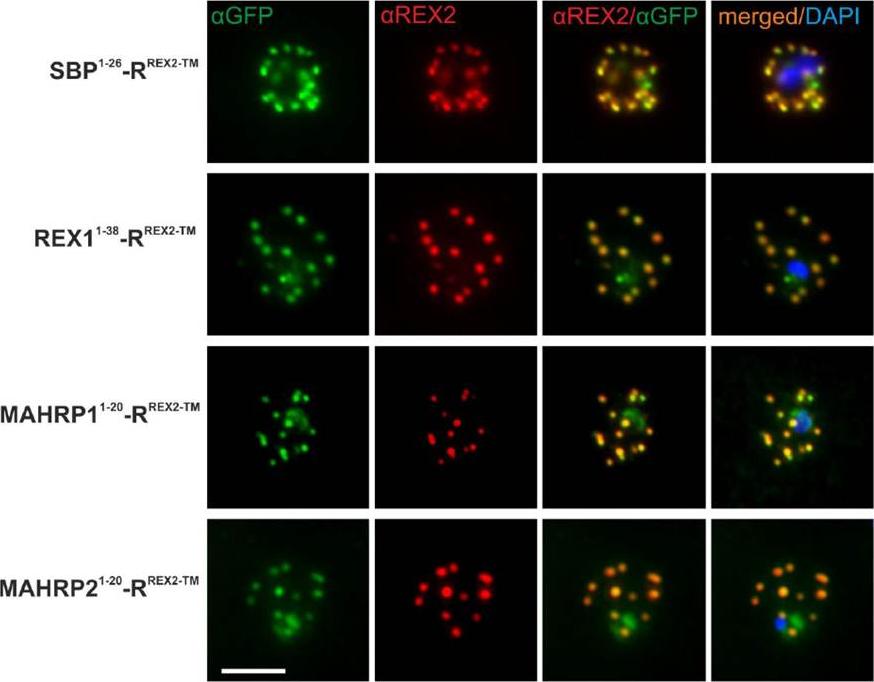
Immunofluorescence Assays Confirm Maurer’s Clefts Localization of PNEP N Termini mTRAP Reporter Fusions. Acetone fixed parasites expressing the reporter indicated on the left were probed with anti-GFP and a serum reacting with the C-terminus of REX2. Under these fixing conditions soluble proteins in the host cell are lost, leading to absence of the soluble pool of the reporter. DIC, differential interference contrast; DAPI, nuclei. Size bar: 5m.Grüring C, Heiber A, Kruse F, Ungefehr J, Gilberger TW, Spielmann T. Development and host cell modifications of Plasmodium falciparum blood stages in four dimensions. Nat Commun. 2011 2:165. PMID:
See original on MMP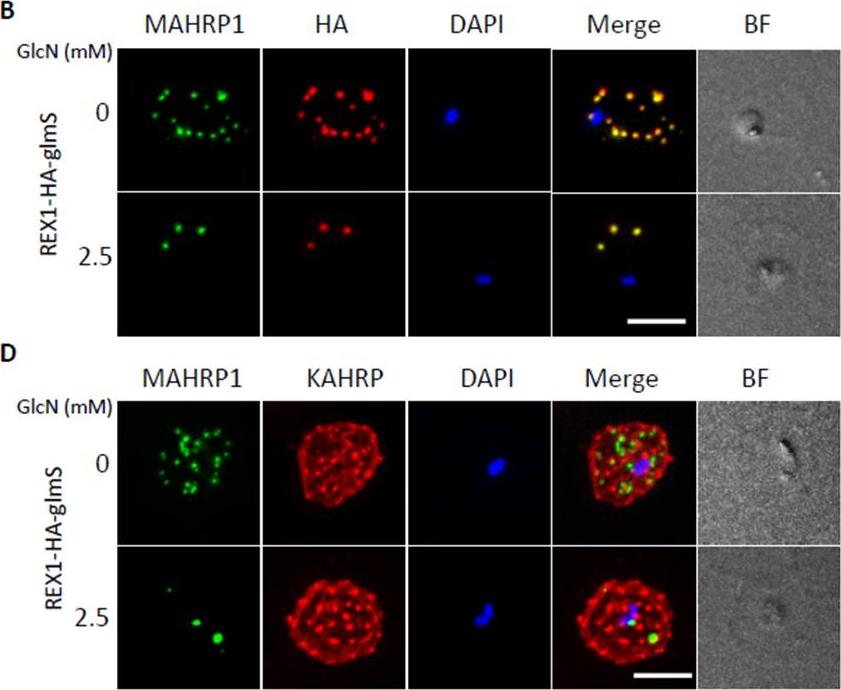
Inducible knockdown of REX1 using the glmS ribozyme system decreases the number of Maurer's cleft puncta. B,D. Immunofluorescencemicroscopy of paraformaldehyde-fixed infected RBCs probed with Maurer’s cleft marker anti-MAHRP1 (green) and anti-HA or anti-KAHRP (red). Nuclei are stained with DAPI (blue). Scale bar = 3 μm. C. Quantitation of numbers of Maurer’s clefts produced by REX1_KD transfectants in the presence and absence of 2.5 mM GlcN. The mean number of Maurer’s clefts produced per singly nucleated infected RBC was determined by counting SBP1-labelled puncta in at least 10 cells. Error bars = S.D. These puncta correspond to individual Maurer’s cleft cisternae that have a distributed organization within the RBC cytoplasmMcHugh E, Batinovic S, Hanssen E, McMillan PJ, Kenny S, Griffin MD, Crawford S, Trenholme KR, Gardiner DL, Dixon MW, Tilley L. A repeat sequence domain of the ring-exported protein-1 of Plasmodium falciparum controls export machineryarchitecture and virulence protein trafficking. Mol Microbiol. 2015 Aug 24. [Epub ahead of print]
See original on MMP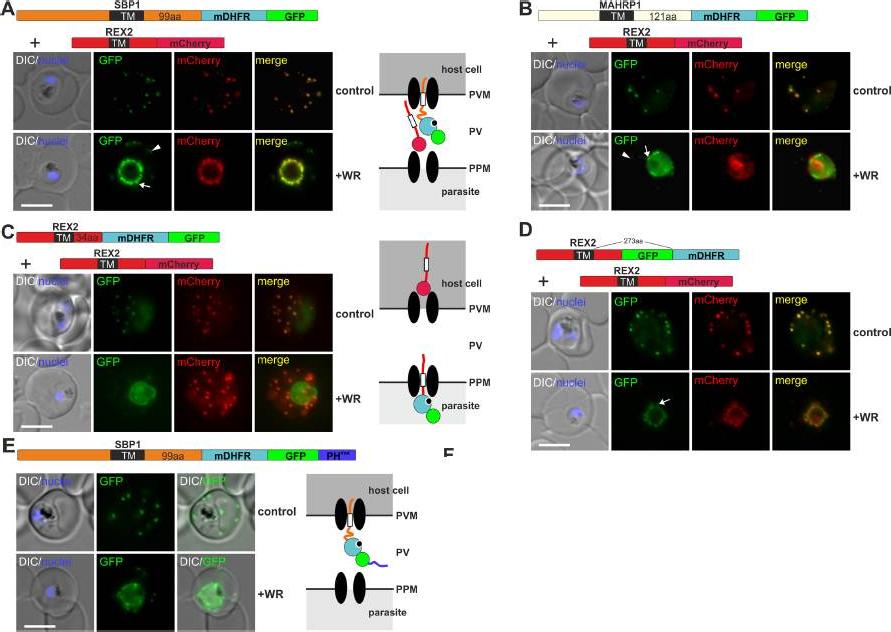
Mouse DHFR fusions can clog the PVM translocon and co-block the export of other proteins. (A-E) Representative images of live P. falciparum parasites grown in the presence (+WR) or absence of WR (control) and expressing the constructs shown schematically above each panel. DIC, differential interference contrast. Arrowheads indicate faint signals at the Maurer’s clefts; arrows show mobile protrusions (note that they do not overlap in the red and the green signal due to movement between capture of the images). Size bars: 5 μm. Schematics to the right show the location of the fusion protein containing the folded WR-bound mDHFR domain (light blue circle with smaller black circle in binding pocket) and the co-expressed REX2 (red line) fused to mCherry (red circle); white box, TM; green circle, GFP; blue line, protease sensitive mutated PH domain.Mesén-Ramírez P, Reinsch F, Blancke Soares A, Bergmann B, Ullrich AK, Tenzer S, Spielmann T. Stable Translocation Intermediates Jam Global Protein Export in Plasmodium falciparum Parasites and Link the PTEX Component EXP2 withTranslocation Activity. PLoS Pathog. 2016 May 11;12(5):e1005618.
See original on MMP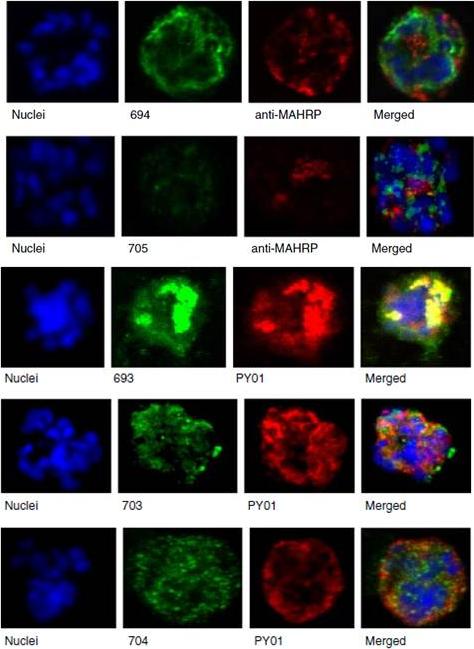
Upper panel: Mouse antisera against MAHRP1 was incubated with antisera against PfMC-2TM peptides and antisera. Lower panel: Immunofluorescence assay of mouse antisera against Rhop-3 (PY01) was incubated with rabbit antibodies against PfMC-2TM on smears of mature schizonts;Tsarukyanova I, Drazba JA, Fujioka H, Yadav SP, Sam-Yellowe TY. Proteins of the Plasmodium falciparum two transmembrane Maurer's cleft protein family, PfMC-2TM, and the 130 kDa Maurer's cleft protein define different domains of the infected erythrocyte intramembranous network. Parasitol Res. 2009 104(4):875-91.
See original on MMP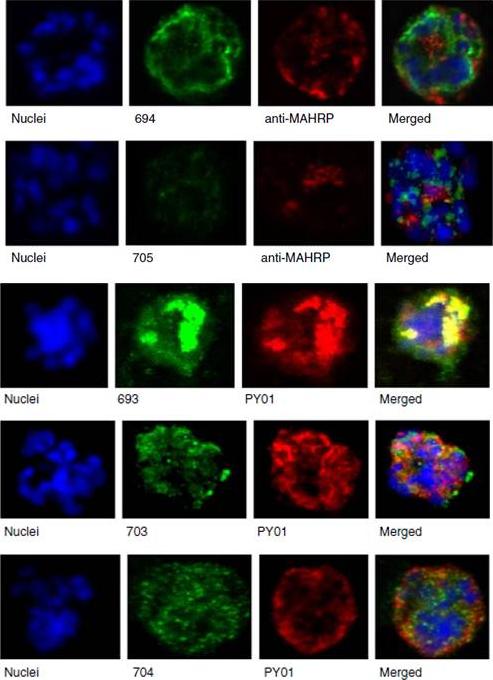
Upper panel: Mouse antisera against MAHRP1 was incubated with antisera against PfMC-2TM peptides and antisera. Lower panel: Immunofluorescence assay of mouse antisera against Rhop-3 (PY01) was incubated with rabbit antibodies against PfMC-2TM on smears of mature schizonts;Tsarukyanova I, Drazba JA, Fujioka H, Yadav SP, Sam-Yellowe TY. Proteins of the Plasmodium falciparum two transmembrane Maurer's cleft protein family, PfMC-2TM, and the 130 kDa Maurer's cleft protein define different domains of the infected erythrocyte intramembranous network. Parasitol Res. 2009 104(4):875-91.
See original on MMP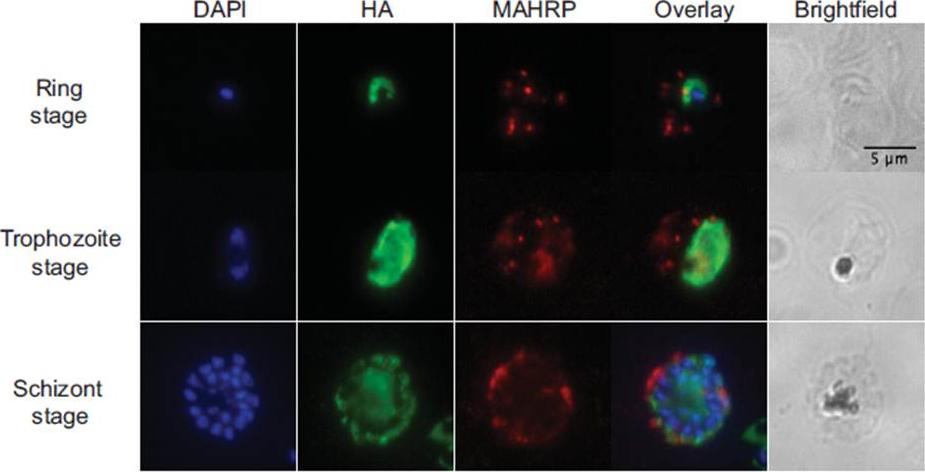
Immunofluorescence. Air-dried thin films of ring (12 hpi), trophozoite (28 hpi) and schizont (44 hpi) stage parasites were fixed in 4% paraformaldehyde, permeabilized with 0.1% Triton X-100, blocked with 3% BSA in phosphate-buffered saline for 1h at RT, incubated with rat α-HA antibody (clone 3F10, 1:50, Roche) at 4oC overnight, and detected with Alexa488-conjugated goat α-rat antibody (1:1000, Invitrogen) for 1h at RT. For PfMAHRP1 co-staining, rabbit α-PfMAHRP1 was added at 1:500 and detected with Alexa555-conjugated goat -rabbit (1:1000, Invitrogen). Slides were mounted with Vectashield anti-fading media containing 4',6-diamidino-2-phenylindole (Vector Laboratories) for nuclear counterstaining. Images were acquired with 100x objectives using a Nikon E800 Microscope. The images were analyzed using ImageJ. PfRACK1 localizes diffusely within the parasite cytoplasm, but not overlapping with the nuclei. we do not observe any exported PfRACK1 in the host cell cytoplasm or in Maurer’s clefts (identified by staining with antibodies directed against PfMAHRP.Blomqvist K, DiPetrillo C, Streva VA, Pine S, Dvorin JD. Receptor for Activated C-Kinase 1 (PfRACK1) is required for Plasmodium falciparum intra-erythrocytic proliferation. Mol Biochem Parasitol. 2016 Oct 9. pii: S0166-6851(16)30129-3.
See original on MMP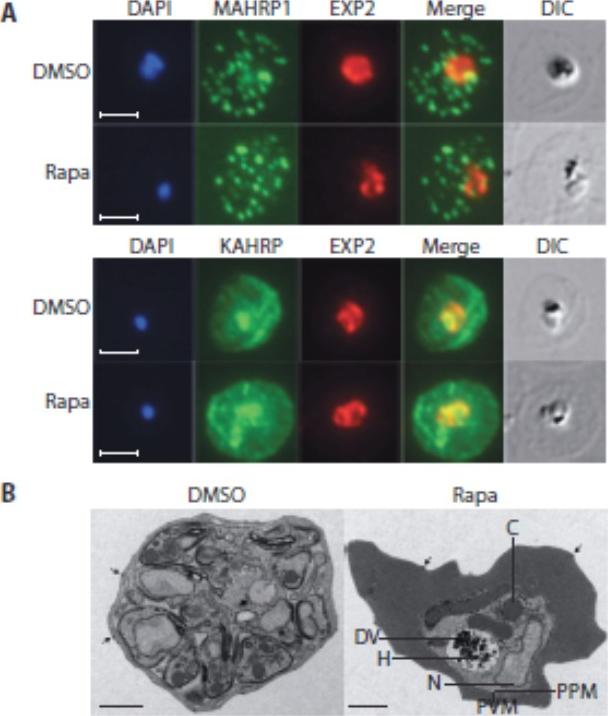
Loss of the RhopH complex does not ablate parasite protein export. Cycle 2 (72 h post rapamycin treatment) DMSO-treated and rapamycintreated rhopH3-loxP clone 5F5 trophozoite-stage parasites were probed withantibodies against the parasitophorous vacuole membrane marker EXP2 to delineate the parasite in the infected erythrocyte, as well as antibodiesspecific for either the Maurer’s cleft marker MAHRP1 (top panels) or the export marker KAHRP (bottom panels). Scale bar, 5 μm. B) Transmission electron micrograph showing a comparison between cycle 2 parasites ofDMSO-treated or rapamycin-treated rhopH3-loxP clone 5F5 parasites ~ 92 h following rapamycin treatment. The developmental block in the RhopH3Δ4-6 parasite is clearly evident, as is the presence of knobs (arrowed) on thesurface of the erythrocyte in both cases. Components of the mutant parasite labelled are the digestive vacuole (DV), haemozoin (H), nucleus (N), parasitophorous vacuole membrane (PVM), cytostomes (C) and parasite plasma membrane (PPM). The mutant parasites displayed no obvious ultrastructural differences from wild-type trophozoites at a similar developmental stage (not shown). Scale bar, 1 μm.Sherling ES, Knuepfer E, Brzostowski JA, Miller LH, Blackman MJ, van Ooij C. The Plasmodium falciparum rhoptry protein RhopH3 plays essential roles in host cell invasion and nutrient uptake. Elife. 2017 6. pii: e23239. PMID: 28252384
See original on MMP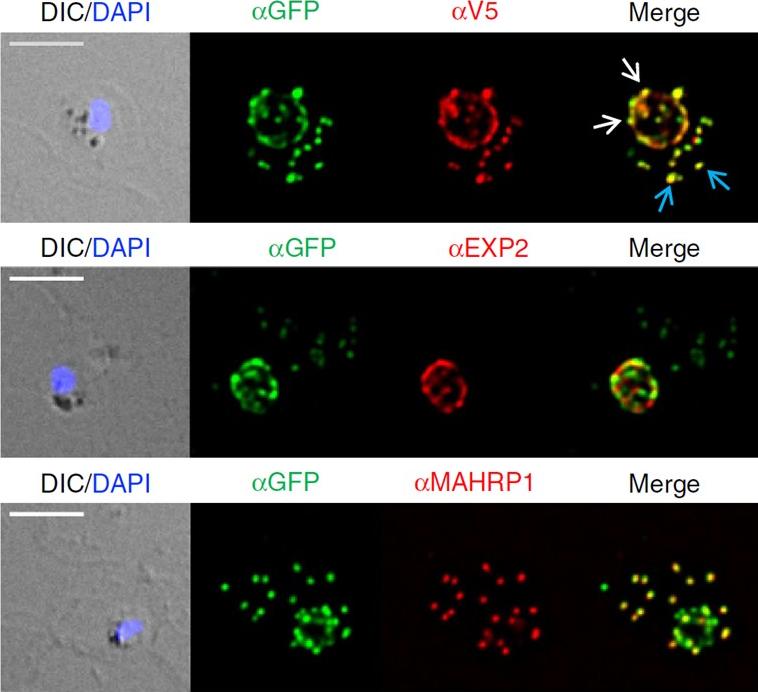
Location and protease accessibility of PfEMP1B during export. Immunofluorescence analysis of PfEMP1B infected RBCs labelled with antisera recognizing GFP (green) and V5 or EXP2 or MAHRP1 (red). The GFP and V5 epitope tags on PfEMP1B are recognized in transgenic parasites expressing the chimera in a compartment with a ‘necklace of beads’ profile at the parasite periphery. Differential interference contrast (DIC) image and parasite nuclei (stained with DAPI; blue) are shown on the left. White arrows indicate PV-localized PfEMP1B while blue arrows indicate PfEMP1B located at the Maurer’s clefts. Scale bars, 5 mm. These compartments respectively represent the PV (where there is partial co-localization with PTEX component exported protein-2 (EXP2)) and the Maurer’s clefts (co-localization with Maurer’s cleft protein membrane-associated histidine-rich protein-1 (MAHRP1)).Batinovic S, McHugh E, Chisholm SA, Matthews K, Liu B, Dumont L, Charnaud SC, Schneider MP, Gilson PR, de Koning-Ward TF, Dixon MWA, Tilley L. An exported protein-interacting complex involved in the trafficking of virulence determinants in Plasmodium-infected erythrocytes. Nat Commun. 2017 10;8:16044.
See original on MMPMore information
| PlasmoDB | PF3D7_1370300 |
| GeneDB | PF3D7_1370300 |
| Malaria Metabolic Pathways | Localisation images Pathways mapped to |
| Previous ID(s) | MAL13P1.413 |
| Orthologs | |
| Google Scholar | Search for all mentions of this gene |