PF3D7_1246200 actin I (ACT1)
Disruptability [+]
| Species | Disruptability | Reference | Submitter | |
|---|---|---|---|---|
| P. falciparum 3D7 |
Refractory |
USF piggyBac screen (Insert. mut.) | USF PiggyBac Screen | |
| P. berghei ANKA |
Refractory |
PlasmoGEM (Barseq) | PlasmoGEM | |
Mutant phenotypes [+]
| Species | Stage | Phenotype | Reference | Submitter |
|---|---|---|---|---|
| P. falciparum 3D7 | Asexual |
Attenuated |
https://bmcbiol.biomedcentral.com/articles/10.1186/s12915-017-0406-2
(Conditional)
DiCre approach. Parasites killed within one replication cycle. Failed apicoplast segmentation and merozoite development. No effect on initial gametocytogenesis. Merozoites are capable of egress but remain attached to food vacuole. No invasion, despite microneme discharge. |
Theo Sanderson, Wellcome Trust Sanger Institute |
Imaging data (from Malaria Metabolic Pathways)
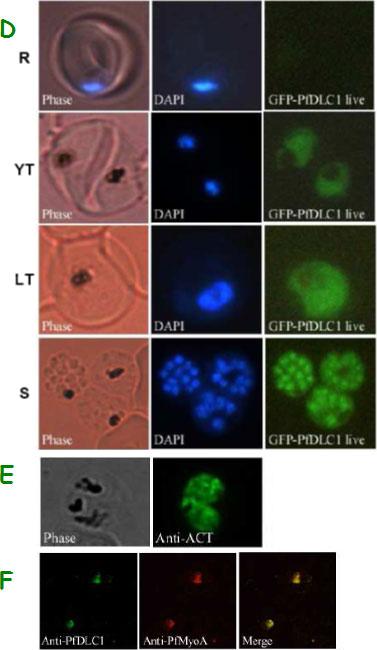
Expression of PfDLC1, PfACT1, and PfMyoA gene products by wild type and transfected P. falciparum. D. Expression and localization of PfDLC1-GFP throughout the erythrocytic cell cycle of P. falciparum. Live transfected parasites were analysed by fluorescence microscopy. E. Immunofluorescence localization of P. falciparum actin in fixed transgenic parasites (late trophozoites). F. Co-localization of PfDLC1 and PfMyoA. Smears of erythrocytes infected with 3D7 strain were fixed and probed with rat anti-PfDLC1 followed by anti-rat IgG-FITC and rabbit anti-PfMyoA, followed by anti-rabbit IgG-Alexa Fluor 568 (red). PfDLC1 location showed a homogenous distribution in the cytoplasm of the parasite (D). It seems that the protein is not exported to the nucleus of P. falciparum or to the RBC cytoplasm or to the RBC surface (Fig. 7C and 7D). The expression of PfDLC1 in younger stage transfectant as well as in the wild type stage (young ring) was undetectable (D). P. falciparum actin is distributed in the parasite cytoplasm (E). Myosin A partially overlap PfDLC1 in schizonts (F).Daher W, Pierrot C, Kalamou H, Pinder JC, Margos G, Dive D, Franke-Fayard B, Janse CJ, Khalife J. Plasmodium falciparum dynein light chain 1 interacts with actin/myosin during blood stage development. J Biol Chem. 2010 285:20180-20191.
See original on MMP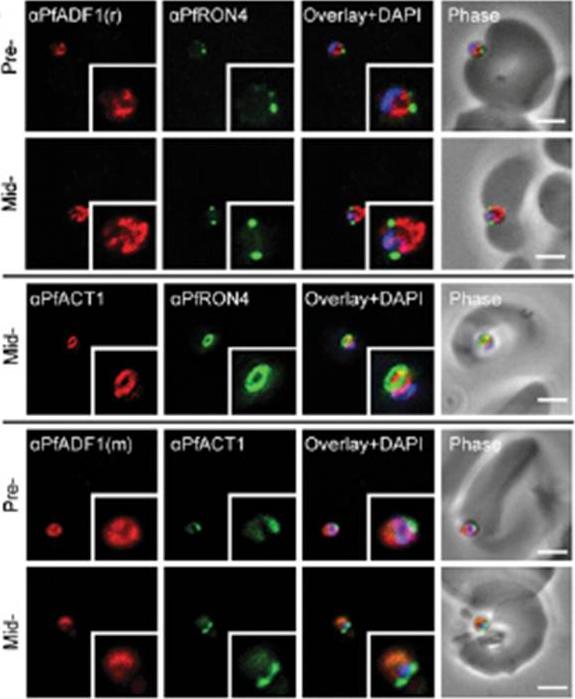
Expression and localization of PfADF1 and PfAct1 in P. falciparum. IFA of merozoite invasion probed with anti-PfADF1, PfAct1, and PfRON4 during merozoite invasion. (Scale bars, 2 μm.). Free merozoites were captured mid-invasion and colabeled with rhoptry neck protein 4 (RON4), a marker of the tight junction that is the point of close apposition between host and parasite membranes during invasion, the site at which a ring of actin forms, a feature consistent with engagement of the actomyosin motor at the junction. PfADF1 retained its cytosolic localization during invasion with the exception that labeling was absent at the tight junction and its associated actin ring.Wong W, Skau CT, Marapana DS, Hanssen E, Taylor NL, Riglar DT, Zuccala ES, Angrisano F, Lewis H, Catimel B, Clarke OB, Kershaw NJ, Perugini MA, Kovar DR, Gulbis JM, Baum J. Minimal requirements for actin filament disassembly revealed by structural analysis of malaria parasite actin-depolymerizing factor 1. Proc Natl Acad Sci U S A. 2011 108:9869-74.
See original on MMP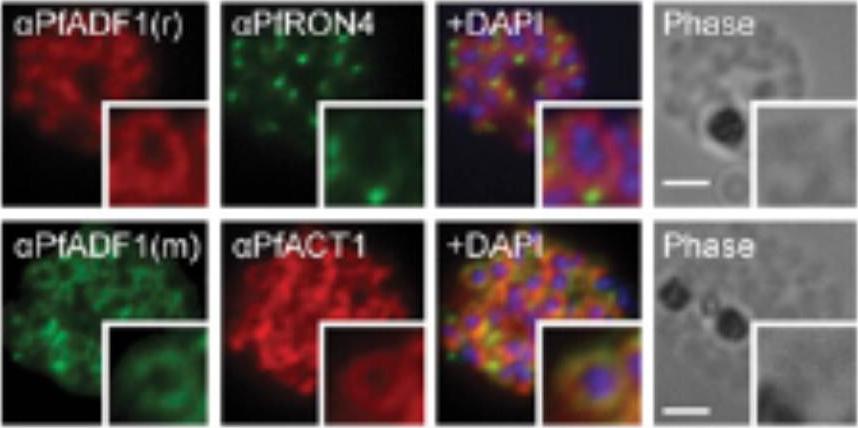
Expression and localization of actin-depolymerizing factor 1 (PfADF1) and actine 1 (PfAct1) in P. falciparum. IFA of mature P. falciparum schizont stages labeled with PfAct1, PfADF1, and PfRON4 antisera. r, rabbit; m, mouse. (Scale bars, 2 μm.) Immunofluorescence assays (IFA) of schizont stages (before merozoite release) demonstrated broad labeling across the parasite cytosol with the exception of the nucleus (marked by DAPI. This distribution was consistent with that of PfAct1.Wong W, Skau CT, Marapana DS, Hanssen E, Taylor NL, Riglar DT, Zuccala ES, Angrisano F, Lewis H, Catimel B, Clarke OB, Kershaw NJ, Perugini MA, Kovar DR, Gulbis JM, Baum J. Minimal requirements for actin filament disassembly revealed by structural analysis of malaria parasite actin-depolymerizing factor 1. Proc Natl Acad Sci U S A. 2011 108:9869-74.
See original on MMP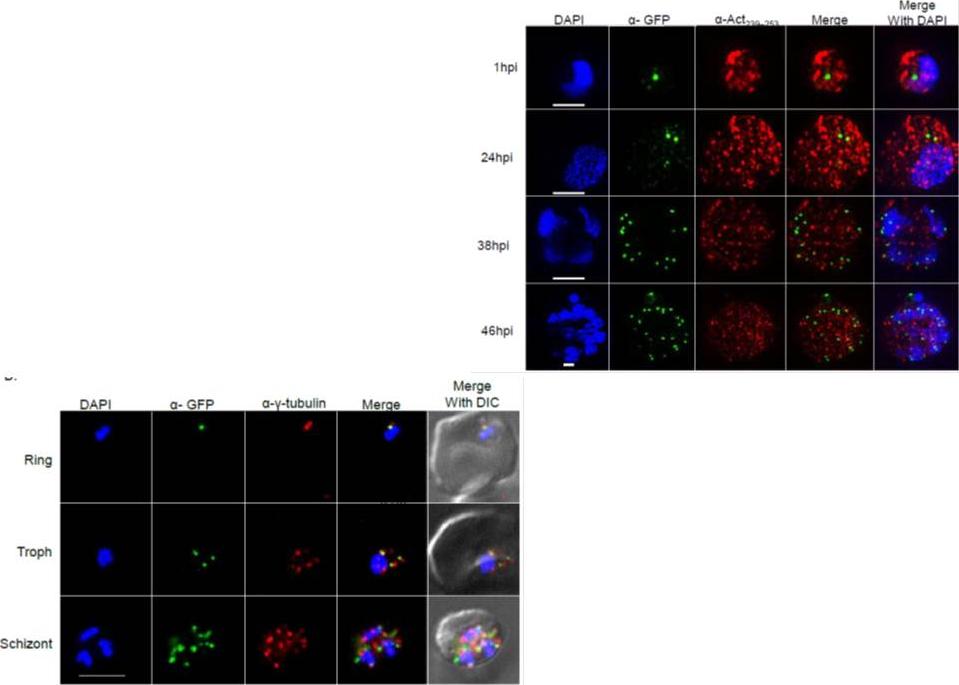
Right: PfSec13 is associated with γ-tubulin rather than with F-actin. Super resolution IFAs of the PfSec13-GFP (green) expressing parasites that were co-stained with F-actin (red) using rabbit α- Act239-253. Nuclei were stained with DAPI (blue). The size bar represents 1μm. (B), IFAs showing co-localization of PfSec13–GFP (green) and γ-tubulin (red) during the IDC. later in the IDC PfSec13 remains located at the nuclear periphery while F-actin is spread throughout the entire cell .Left: PfSec13 is associated with γ-tubulin rather than with F-actin. IFAs showing co-localization of PfSec13–GFP (green) and γ-tubulin (red) during the IDC. PfSec13 is associated with F-actin at the nuclear periphery. The association of PfSec13 with γ-tubulin may indicate a possible role of microtubules in NPCs dynamics.Dahan-Pasternak N, Nasereddin A, Kolevzon N, Pe'er M, Wong W, Shinder V, Turnbull L, Whitchurch CB, Elbaum M, Gilberger TW, Yavin E, Baum J, Dzikowski R. PfSec13 is an unusual chromatin associated nucleoporin of Plasmodium falciparum, which is essential for parasite proliferation in human erythrocytes. J Cell Sci. 2013 126(Pt 14):3055-69
See original on MMP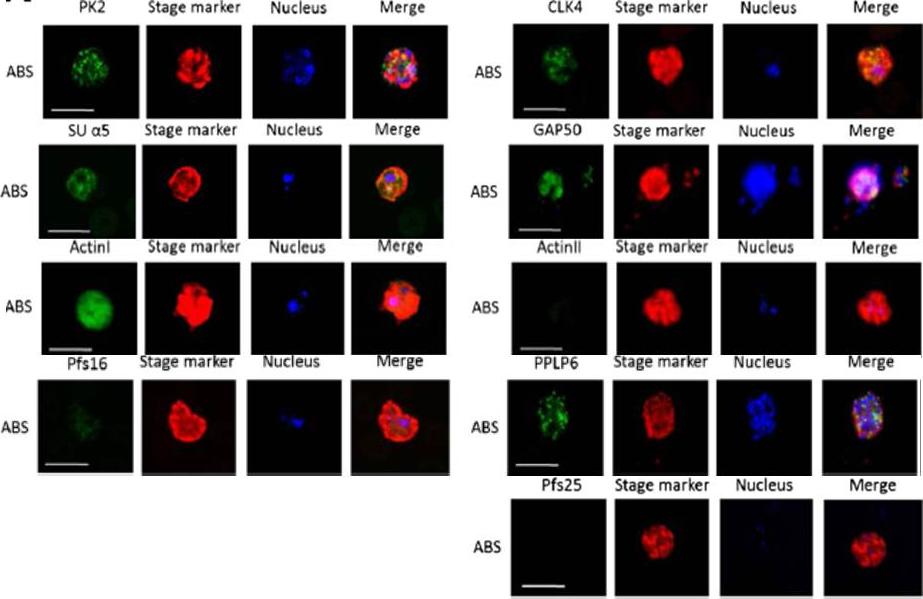
Immunofluorescence assays, using specific antibodies, detected the proteins of interest (in green) in asexual blood stage (ABS) parasites, using MSP1 (in red). Nuclei were highlighted by Hoechst nuclear stain (in blue). Bar, 5 μm. Ngwa CJ, Scheuermayer M, Mair GR, Kern S, Brügl T, Wirth CC, Aminake MN, Wiesner J, Fischer R, Vilcinskas A, Pradel G. Changes in the transcriptome of the malaria parasite Plasmodium falciparum during the initial phase of transmission from the human to the mosquito. BMC Genomics. 2013 Apr 15;14:256
See original on MMP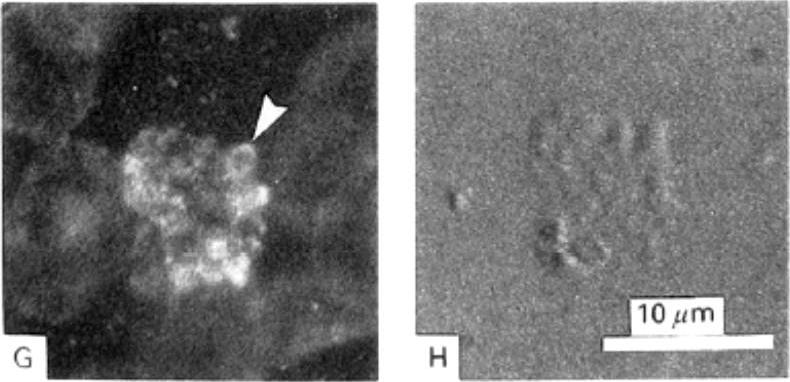
Detection of actin in blood stage parasites by fluorescent staining techniques. A) Image of a mature schizont and released merozoites stained with TRITC-phalloidin showing brighter polar fluorescence of the merozoites (Δ). B) The corresponding DIC image shows the bright contrast image of malarial pigment. G) Fluorescence associated with mature and rupturing schizont and with the membranes of all erythrocytes after immuno-staining using a monoclonal AB to actin. Merozoite-associated fluorescence was strongest in the periphery (Δ). H) Corresponding DIC image.Webb SE, Fowler RE, O'Shaughnessy C, Pinder JC, Dluzewski AR, Gratzer WB, Bannister LH, Mitchell GH. Contractile protein system in the asexual stages of the malaria parasite Plasmodium falciparum. Parasitology. 1996 112:451-7.
See original on MMP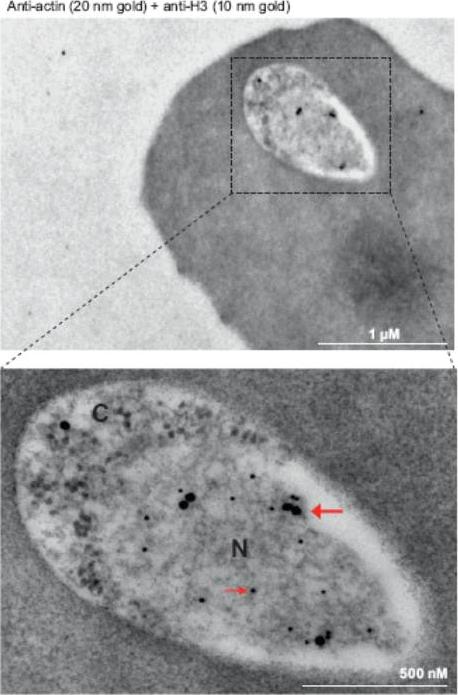
Immunoelectron microscopy of 3D7 rings. Double labeling with mouse anti-Pfactin-I (20 nm gold grain, bold arrow) and rabbit anti-histone H3 (10 nm gold grain, slim arrow). N, nucleus; C, cytoplasm. Gold grains are virtually confined to the nuclear periphery.Zhang Q, Huang Y, Zhang Y, Fang X, Claes A, Duchateau M, Namane A, Lopez-Rubio JJ, Pan W, Scherf A. A critical role of perinuclear filamentous actin in spatial repositioning and mutually exclusive expression of virulence genes in malaria parasites. Cell Host Microbe. 2011 10:451-63.
See original on MMP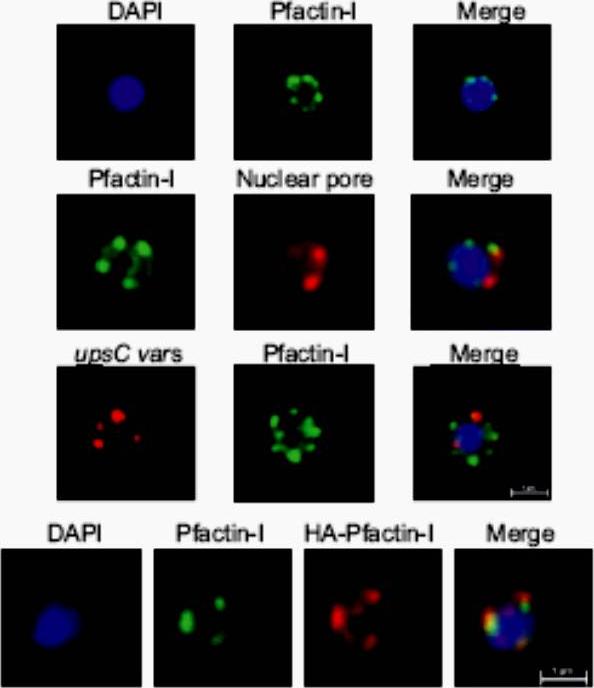
Upper panel: IFA visualization of subcellular actin in rings using an anti-Pfactin-I antibody (rabbit #2). Second panel: Two-color IFA assay using anti-Pfactin-I (rabbit #2, green) and anti-nuclear pore (PF14_0706) antibody (rat, red). Third panel: Combined FISH/IFA assay in rings showing partial colocalization of chromosome internal var genes (upsC-type) and actin. Fourth panel: IFA analysis of HA-actin-transfected ring stage parasites using mouse anti-HA antibody (red) and anti-Pfactin-I antibody (rabbit #2, green). IFA analysis with anti-Pfactin-I antibodies or with anti-HA antibody in HAactin-transfected 3D7 parasites confirmed the restricted location of actin to the nucleus in ring stage parasites and demonstrated that it concentrated in foci at the nuclear periphery. Colocalization with a nuclear pore antibody indicates that the staining is limited to the inner side of the nucleus.Zhang Q, Huang Y, Zhang Y, Fang X, Claes A, Duchateau M, Namane A, Lopez-Rubio JJ, Pan W, Scherf A. A critical role of perinuclear ilamentous actin in spatial repositioning and mutually exclusive expression of virulence genes in malaria parasites. Cell Host Microbe. 2011 10:451-63.
See original on MMP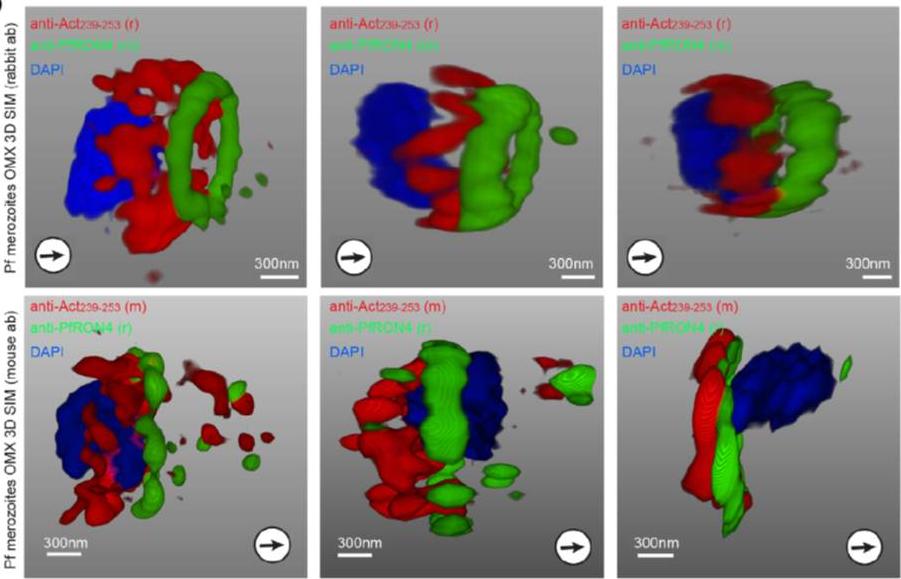
3D structured illumination microscopy (3D SIM) of three separate invading P. falciparum merozoites labelled with rabbit (upper row) and mouse (lower row) anti-Act239–253. Labelling shows actin (Red), RON4 (Green) and DAPI (Blue). In all instances Anti-Act239–253 labelling was concentrated in a ring lying posterior to the tight junction during merozoite invasion, defined as the edge of the junction towards the posterior of the parasite.Angrisano F, Riglar DT, Sturm A, Volz JC, Delves MJ, Zuccala ES, Turnbull L, Dekiwadia C, Olshina MA, Marapana DS, Wong W, Mollard V, Bradin CH, Tonkin CJ, Gunning PW, Ralph SA, Whitchurch CB, Sinden RE, Cowman AF, McFadden GI, Baum J. Spatial Localisation of Actin Filaments across Developmental Stages of the Malaria Parasite. PLoS One. 2012;7(2):e32188.
See original on MMP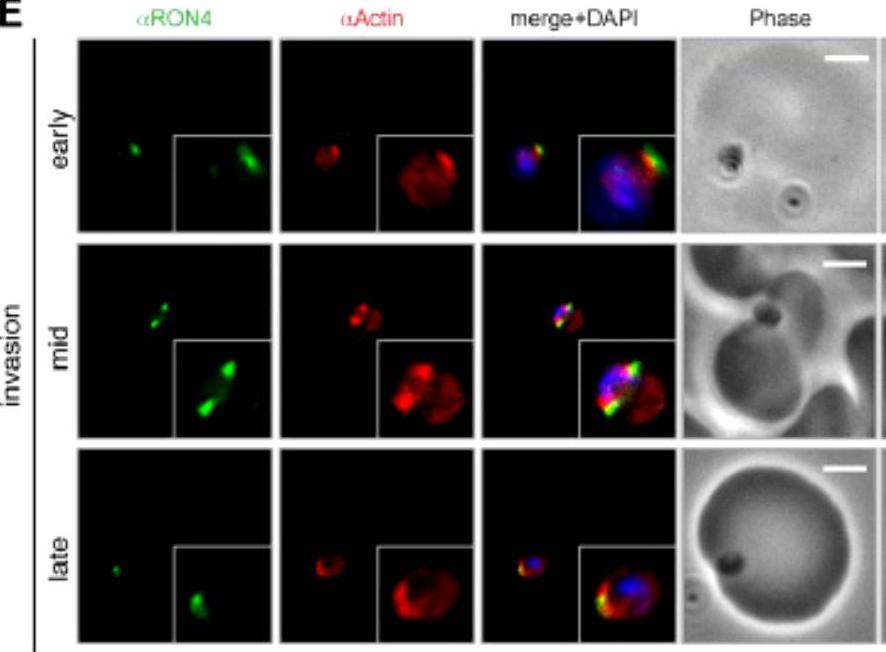
Wide field IFA time course of invasion using anti-PfActin/PfRON4. IFA scale bar = 2.0 μm. The distribution of actin within the merozoite was concentrated at the parasite apex, with diffuse labeling elsewhere in the cytoplasm (Actin early). Actin fluorescence could be detected, concentrated at the tight junction (Actin mid). In the latter stages of invasion, the concentration of actin labeling was at the rear of the invaded merozoite (Actin late).Riglar DT, Richard D, Wilson DW, Boyle MJ, Dekiwadia C, Turnbull L, Angrisano F, Marapana DS, Rogers KL, Whitchurch CB, Beeson JG, Cowman AF, Ralph SA, Baum J. Super-resolution dissection of coordinated events during malaria parasite invasion of the human erythrocyte. Cell Host Microbe. 2011 9:9-20.
See original on MMP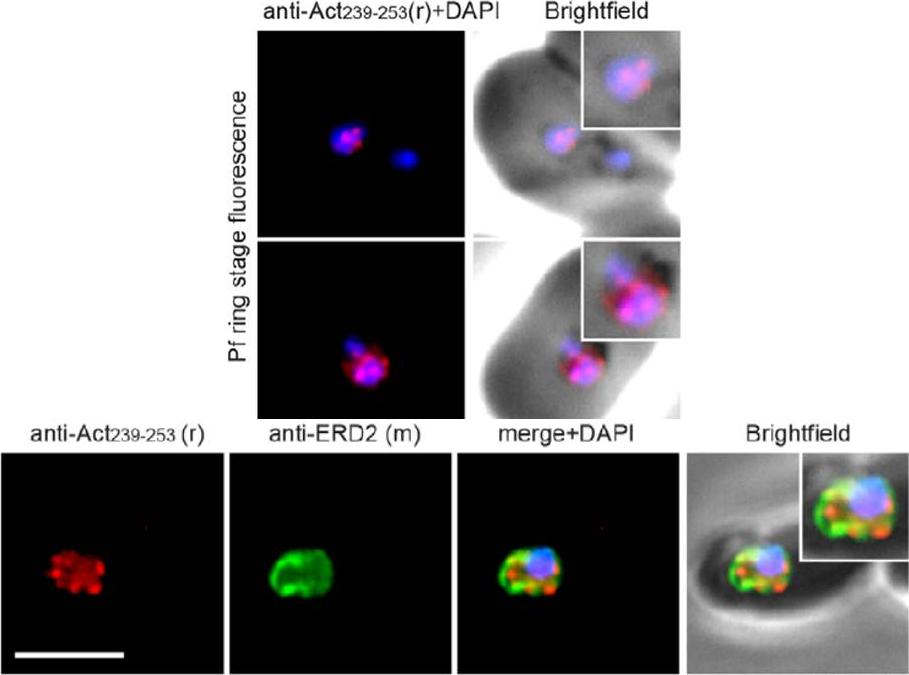
Upper panel: Widefield IFA of P. falciparum rings labelled with rabbit anti-Act239–253 (Red) and DAPI (Blue). Early ring stage asexual parasites were labelled with anti-actin and the nuclear marker DAPI. Very early rings demonstrated a consistent punctate labelling of actin within DAPI staining of the nucleus.Lower panel: Two colour widefield IFA using rabbit anti-Act239–253 (Red), rat anti-ERD2 (Green) and DAPI (Blue) Scale bar = 5 mm. The concentration of stabilised actin filaments at the nuclear periphery was confirmed using ERD2, a cis-Golgi marker that localises to defined sites adjacent to the nucleusAngrisano F, Riglar DT, Sturm A, Volz JC, Delves MJ, Zuccala ES, Turnbull L, Dekiwadia C, Olshina MA, Marapana DS, Wong W, Mollard V, Bradin CH, Tonkin CJ, Gunning PW, Ralph SA, Whitchurch CB, Sinden RE, Cowman AF, McFadden GI, Baum J. Spatial Localisation of Actin Filaments across Developmental Stages of the Malaria Parasite. PLoS One. 2012;7(2):e32188.
See original on MMP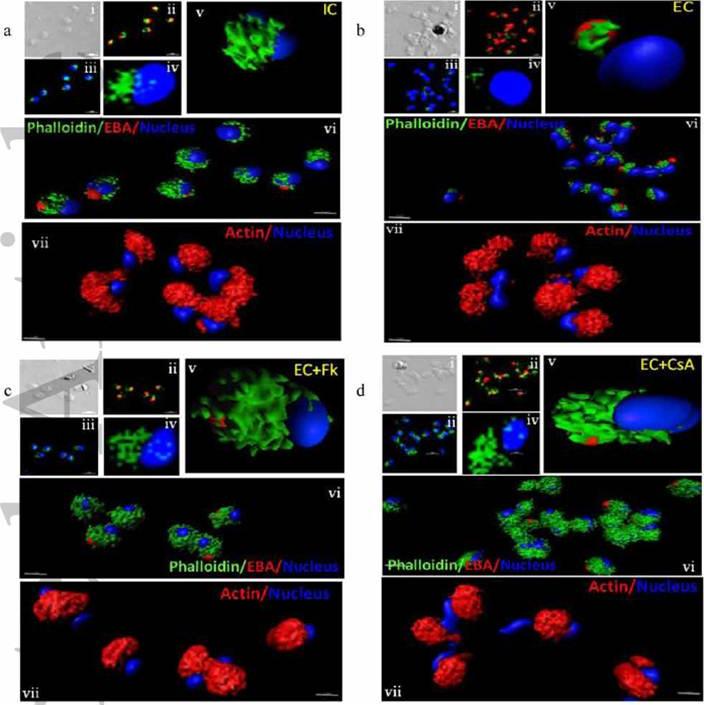
Actin polymerization status in P. falciparum merozoites. Merozoites were stained with Alexa 488-labeled phalloidin to detect polymerized F-actin (green), mouse anti EBA-175 mouse sera and Alexa Fluor 594-conjugated anti-mouse IgG goat antibodies to define location of micronemes (red) and DAPI to define location of nuclei (blue). Slides were observed under a confocal laser microscope (Nikon A1R). Bright field (i) and single slice confocal fluorescence images of merozoites (ii – iv) are shown. Three-dimensional reconstruction of confocal z stack fluorescence images of merozoites was performed using Imaris software (v –vii). Total actin (polymerized F-actin and monomeric G-actin) was detected with anti-actin rabbit sera followed by Alexa Fluor 594-conjugated anti-rabbit IgG goat antibodies (red in vii). Levels of total actin are similar in all conditions tested. Transfer of merozoites from IC to EC buffer leads to disassembly of polymerized F-actin at the apical tip of merozoites. Treatment of merozoites with FK506 and CsA prior to transfer to EC buffer leads to accumulation of polymerized actin at the apical end. Actin depolymerizing agent CytD and actin stabilizing agent JAS were used as control and result in reduced F-actin and increased F-actin at the apical tip of the merozoites respectively. Scale bar represents 1 μm. Treatment of merozoites with actin depolymerizing agents, CytD, ML-B and LA-B, resulted in increased secretion of microneme protein PfAMA-1 (a-c) whereas treatment with polymerized actin stabilizer JAS resulted in reduced secretion of PfAMA-1 (d).Singh S, More KR, Chitnis CE. Role of Calcineurin and Actin Dynamics in Regulated Secretion of Microneme Proteins in Plasmodium falciparum Merozoites during Erythrocyte Invasion. Cell Microbiol. 2013 Jun;21(2):125-7
See original on MMP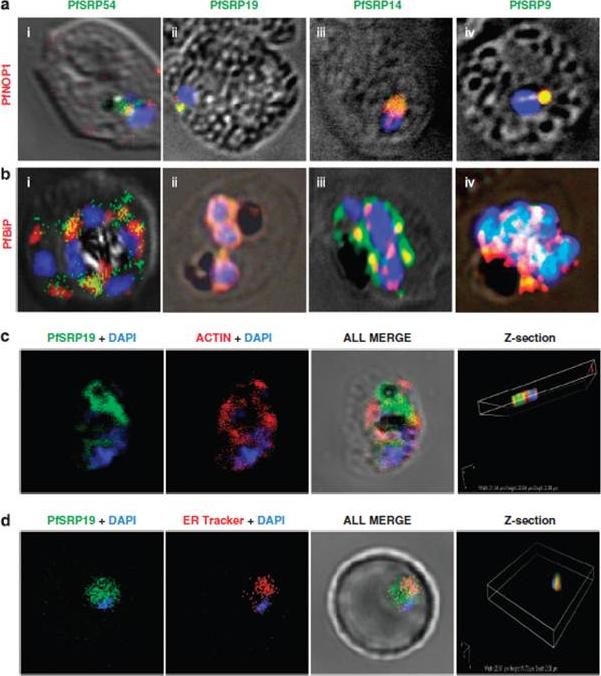
Co-staining of PfSRP polypeptides with nucleolar marker PfNOP1, ER marker PfBiP and cytoplasmic marker Pfactin. (a, b and c) P. falciparum infected erythrocytes stained with anti-PfSRP antibodies (green), anti-PfNOP1 (red), anti-PfBiP (red) and anti-Pfactin (red) respectively. (d) localization of GFP fused SRP19 (green) with ER tracker (red). DAPI is used for nuclear staining (blue). A considerable overlap in staining was observed between three PfSRP polypeptides; PfSRP19, -14, and -9 with PfNOP1 and with PfBiP protein (a and b, i-iv). Importantly, considerable co-localization of PfSRP54 with PfNOP1 in early stages of parasite development i.e. merozoite and ring stages.Panchal M, Rawat K, Kumar G, Kibria KM, Singh S, Kalamuddin M, Mohmmed A, Malhotra P, Tuteja R. Plasmodium falciparum signal recognition particle components and anti-parasitic effect of ivermectin in blocking nucleo-cytoplasmic shuttling of SRP. Cell Death Dis. 2014 Jan 16;5:e994.
See original on MMP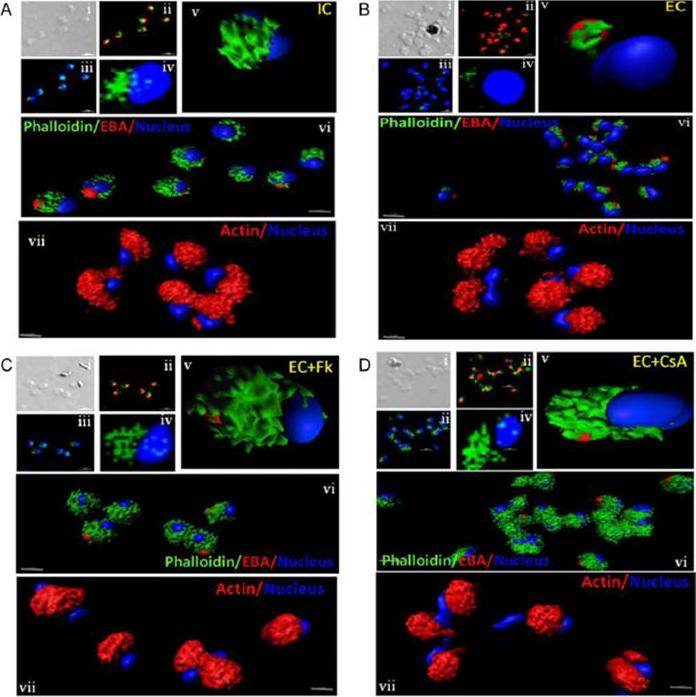
Actin polymerization status in P. falciparum merozoites. Merozoites were stained with Alexa 488-labeled phalloidin to detect polymerized F-actin (green), mouse anti EBA-175 mouse sera and Alexa Fluor 594-conjugated anti-mouse IgG goat antibodies to define location of micronemes (red) and DAPI to define location of nuclei (blue). Slides were observed under a confocal laser microscope (Nikon A1R). Bright field (i) and single slice confocal fluorescence images of merozoites (ii – iv) are shown. Three-dimensional reconstruction of confocal z stack fluorescence images of merozoites was performed using Imaris software (v –vii). Total actin (polymerized F-actin and monomeric G-actin) was detected with anti-actin rabbit sera followed by Alexa Fluor 594-conjugated anti-rabbit IgG goat antibodies (red in vii). Levels of total actin are similar in all conditions tested. Transfer of merozoites from IC to EC buffer leads to disassembly of polymerized F-actin at the apical tip of merozoites. Treatment of merozoites with FK506 and CsA prior to transfer to EC buffer leads to accumulation of polymerized actin at the apical end. Actin depolymerizing agent CytD and actin stabilizing agent JAS were used as control and result in reduced F-actin and increased F-actin at the apical tip of the merozoites respectively. Scale bar represents 1 μm. Treatment of merozoites with actin depolymerizing agents, CytD, ML-B and LA-B, resulted in increased secretion of microneme protein PfAMA-1 (a-c) whereas treatment with polymerized actin stabilizer JAS resulted in reduced secretion of PfAMA-1 (d).Singh S, More KR, Chitnis CE. Role of Calcineurin and Actin Dynamics in Regulated Secretion of Microneme Proteins in Plasmodium falciparum Merozoites during Erythrocyte Invasion. Cell Microbiol. 2013 16(1), 50–63
See original on MMP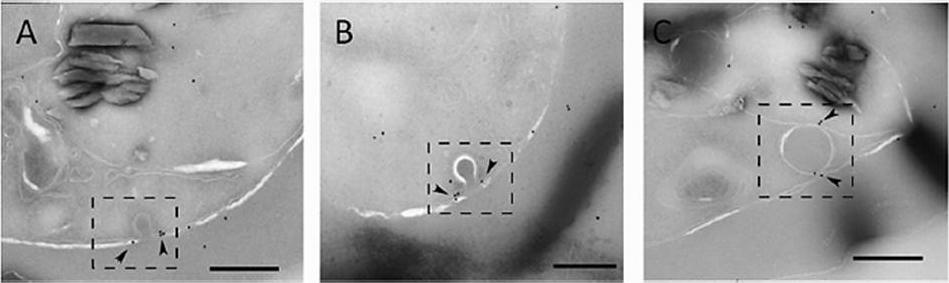
Actin localizes to the cytostome neck in IRBC. Representative immunoelectron micrographs of P. falciparum trophozoites probed with anti-T. gondii actin antibody. (A and B) Micrographs showing actin labeled with 12-nm gold particles (arrowheads) localized to electron-dense collars at the cytostome neck (dashed box). (C) Actin (arrowheads) associated with a hemoglobin-containing compartment in the parasite cytosol (dashed box). Scale bars, 250 nm.Milani KJ, Schneider TG, Taraschi TF. Defining the Morphology and Mechanism of the Hemoglobin Transport Pathway in Plasmodium falciparum-Infected Erythrocytes. Eukaryot Cell. 2015 14(4):415-26. PMID:
See original on MMP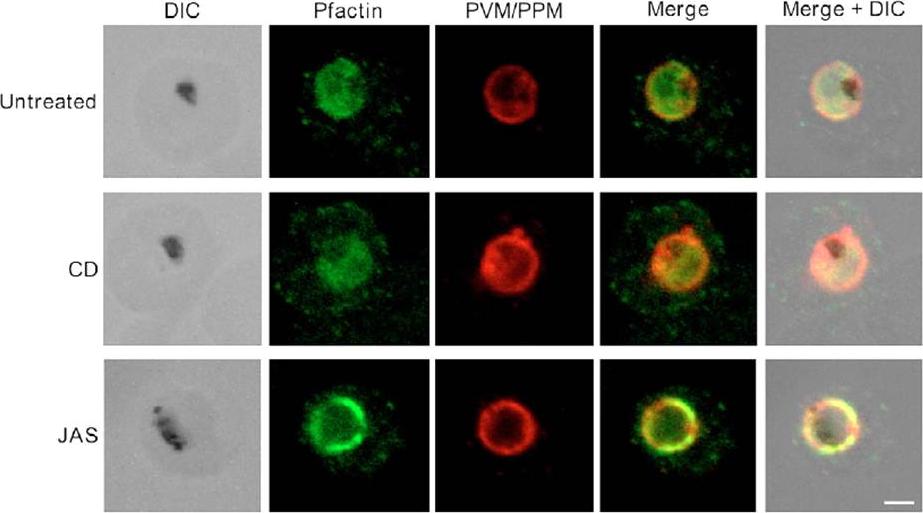
Pfactin distribution and localization in untreated, JAS- or CD-treated trophozoite stage PE. Representative confocal microscopy images showing Pfactin localization (green) in relation to the PVM/PPM (red) in untreated, JAS- and CD-treated trophozoite PE. Merge is a composite of the green and red images; merged+DIC is a composite of the DIC, green and red images. The electron-dense inclusion (hemozoin) in the DIC images indicates the location of the parasite FV. Bar, 2.0 μm.Lazarus MD, Schneider TG, Taraschi TF. A new model for hemoglobin ingestion and transport by the human malaria parasite Plasmodium falciparum. J Cell Sci. 2008 Jun 1;121(Pt 11):1937-49. PMID:
See original on MMP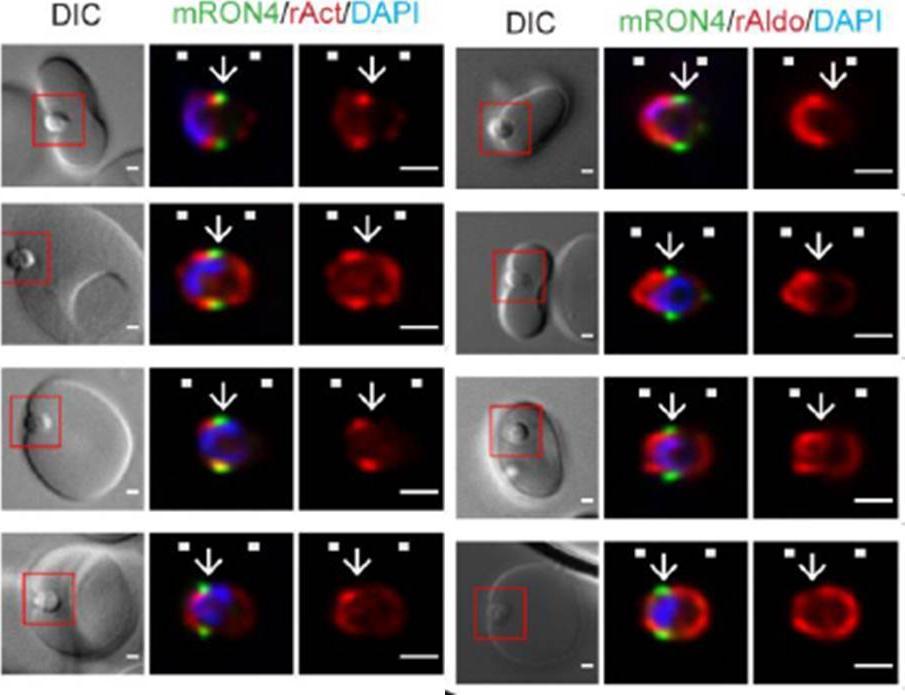
Longitudinal intensity profiling localises actin and aldolase towards the rear of the invading merozoite’s tight junction. Single-slice images of example merozoites for the longitudinal intensity profiling of mRON4 (green) vs (left panel) rActin (rAct) (red) or (right panel) rAlodlase (rAldo) (red). Scale bars = 1 μm. Red boxes in DIC images indicate zoomed regions for middle and right panels. Arrows indicate the position of the RON4 labelled tight junctionRiglar DT, Whitehead L, Cowman AF, Rogers KL, Baum J. Localization-based imaging of malarial antigens during red cell entry reaffirms role for AMA1 but not MTRAP in invasion. J Cell Sci. 2015. [Epub ahead of print] PMID:
See original on MMP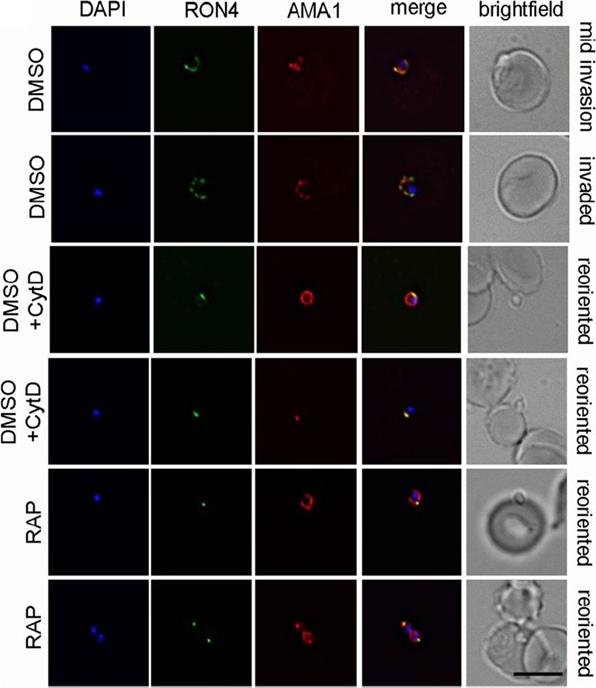
PfACT1 KO merozoites can secrete their micronemes and form a tight junction (TJ) but cannot invade erythrocytes. IFA of TJ formation. Colocalisationof rhoptry neck protein 4 (RON4) and AMA1 at the merozoite-erythrocyte boundary indicates successful TJ formation in DMSO controls (upper twopanels), controls treated with cytochalasin D (middle two panels) and in PfACT1 KOs (lower two panels). Seventy-six percent of DMSO control parasitesinvaded erythrocytes in the time frame of the assa. In contrast, 84% of RAP-treated parasites attached to the erythrocyte and could undergo reorientationand appeared to secrete RON proteins which are required for formation of the junction. However, a typical circular junction could never be observed andparasites were incapable of invading erythrocytes demonstrating a critical requirement for parasite actin for host cell invasion.Das S, Lemgruber L, Tay CL, Baum J, Meissner M. Multiple essential functions of Plasmodium falciparum actin-1 during malaria blood-stage development. BMC Biol. 2017 Aug 15;15(1):70.
See original on MMP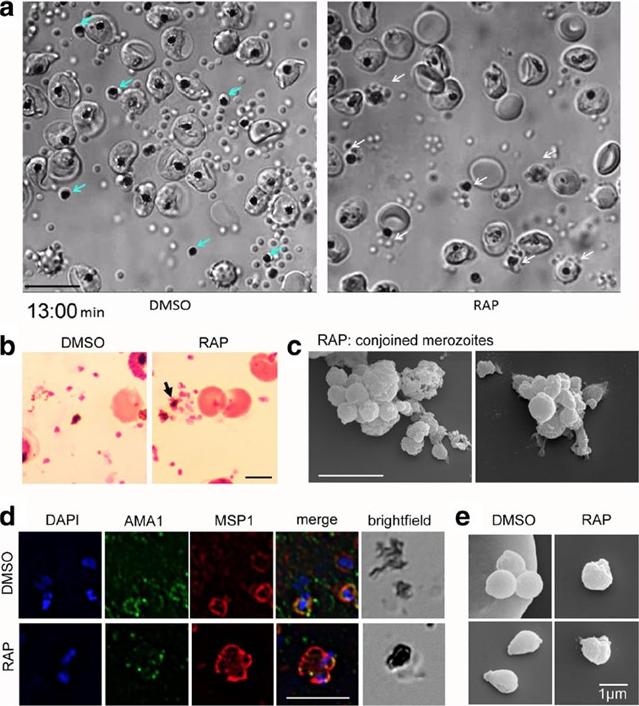
PfACT1 KO merozoites remain conjoined post-egress and possess a dysmorphic ruffled surface. a Still images from Additional file 6: Video S1show normal explosive egress in PfACT1 KOs; however, the RAP-treated population displays conjoined merozoites attached to the FV (white arrows),compared to completely segregated merozoites not attached to the FV (blue arrows) in DMSO controls. b Post-egress, Giemsa-stained RAP-treatedpopulations have parasite structures attached to the FV (black arrow). In DMSO controls, the newly released daughter merozoites are free and notconnected to the FV. Scale bar 5 μm. c Conjoined merozoites with a dysmorphic ruffled surface are apparent by SEM in the RAP-treated population. Scale bar 5 μm. d IFA on post-egress preparations of RAP-treated parasites reveals nuclei (DAPI) and micronemes (AMA1) joined to the FV, with the entire structure bounded by a contiguous plasma membrane (MSP1); defect observed in 76% of all FVs. The FV and merozoites are distinct in DMSO controls (94% of all FVs). N= 21 for DMSO and N = 33 for RAP. Scale bar 5 μm. e Free merozoites are released in the PfACT1 KO population, though they often possess a ruffled surface as observed by SEM. Scale bar 1 μm.Das S, Lemgruber L, Tay CL, Baum J, Meissner M. Multiple essential functions of Plasmodium falciparum actin-1 during malaria blood-stage development. BMC Biol. 2017 Aug 15;15(1):70.
See original on MMP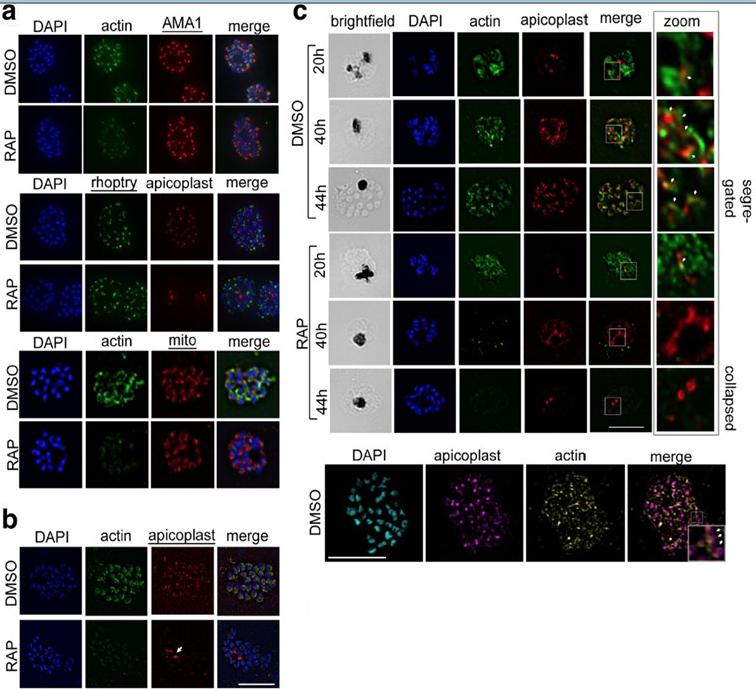
PfACT1 is not required for secretory organelle formation but is crucial for apicoplast segregation. a RAP-treated PfACT1 KO parasites have similar microneme (anti-AMA1), rhoptry (anti-RhopH2) and mitochondria (mito; anti-TOM40) architecture as DMSO controls, as revealed by IFA. b The apicoplast in PfACT1 KO parasites fails to segregate to daughter merozoites and collapses to an amorphous mass close to the food vacuole (white arrow). c IFA of samples drawn at various time points shows that apicoplast reticulation and division increase with nuclear division (DMSO, 20, 40, 44 h). The apicoplast shows close apposition to F-actin staining (zoom, white arrows). Actin staining disappears within 40 h of RAP treatment. The apicoplast does not show reticulation and extensive migration in the absence of PfACT1 (RAP, 40 and 44 h). Scale bar 5 μm. Bottom panel: Super-resolution imaging reveals close apposition of apicoplasts on the actin network. Enlarged inset in ‘merge’ shows apicoplast colocalised to actin filament (white arrows). Colocalisation analysis of apicoplast on actin in the entire image yielded a Manders coefficient of 0.83.Das S, Lemgruber L, Tay CL, Baum J, Meissner M. Multiple essential functions of Plasmodium falciparum actin-1 during malaria blood-stage development. BMC Biol. 2017 Aug 15;15(1):70.
See original on MMP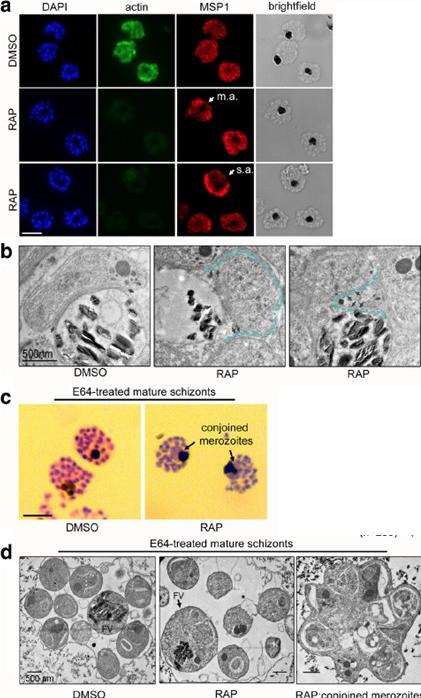
PfACT1 is required for normal cytokinesis. a IFA of PfACT1 KO and control schizonts. Aberrant staining of the plasma membrane marker MSP1 (red) depicts dysmorphic merozoites in the schizonts (white arrow) in the absence of PfACT1 (actin). Scale bar 5 μm. Normal, moderately aberrant (m.a.), and severely aberrant (s.a.) MSP1 staining has been exemplified (white arrows). b TEM on C1-treated mature schizonts stalled just prior to egress. Whilst medially resident daughter merozoites have distinct, separated membranes apposed to the FV membrane in DMSO controls (black arrows), aberrant membranous pockets including merozoite material are observed in RAP-treated parasites (double black arrows and outlined by blue dotted line). c Giemsa-stained, E64-treated mature schizonts show conjoined merozoites (black arrows) with ~50% frequency in the RAP-treated sample as compared to <10% in DMSO controls.. d TEM of E64-trapped merozoites. Merozoites in DMSO controls are distinct and well formed, with organelles not included within the FV membrane (left panel). The RAP-treated population has FVs (black arrow) which include organelles normally resident in daughter merozoites. Some merozoites show aberrant surface architecture (asterisked). Merozoites conjoined to each other are frequently seen in the PfACT1 KO population (right panel). TEM scale bar 500 nm. Other scale bars 5 μm.Das S, Lemgruber L, Tay CL, Baum J, Meissner M. Multiple essential functions of Plasmodium falciparum actin-1 during malaria blood-stage development. BMC Biol. 2017 Aug 15;15(1):70. PMID: 28810863
See original on MMP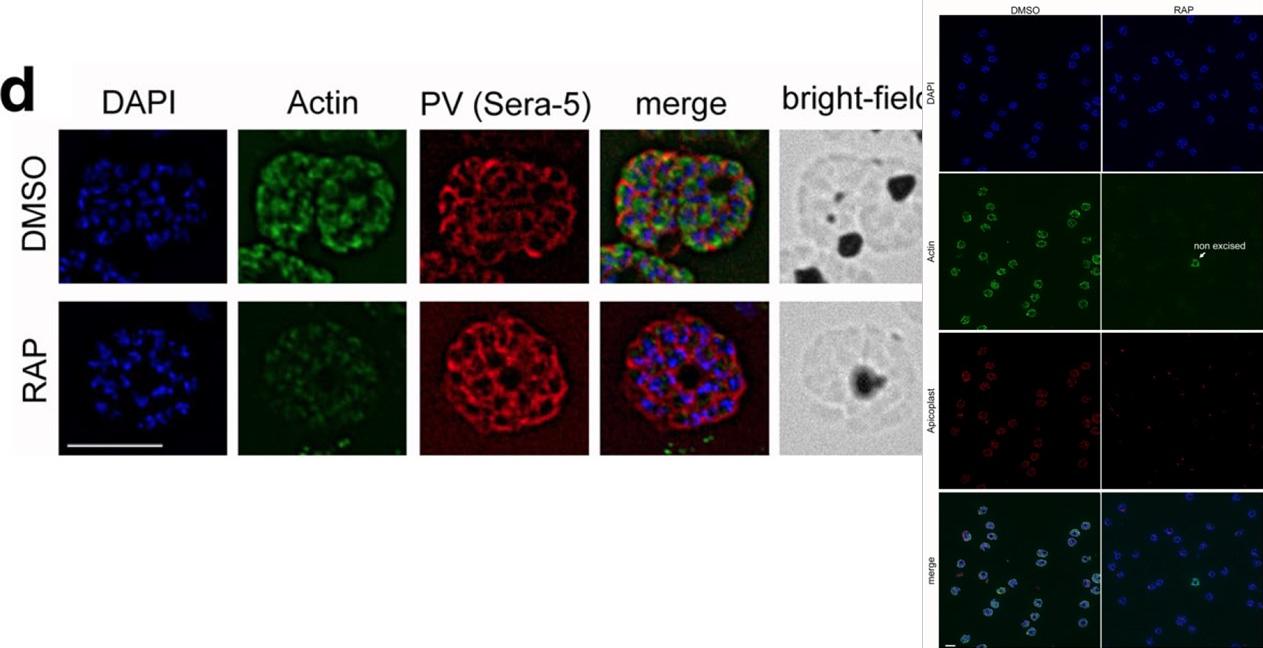
IFA shows a loss of reactivity of PfACT1 KOs to the anti-PfACT1 antibody. The PV is depicted in red (Sera-5). Scale bar 5 μ. PfACT1 disruption was apparent by immunofluorescence assay (IFA) in schizonts 44 h post-induction. Based on IFAs, it was estimated that ~98% of the population had undergone excision of pfact1, resulting in an almost pure population of pfact1 disrupted parasites (PfACT1 KO) for phenotypic analysis. Only a weak, potentially non-specific signal could be detected by IFA in PfACT1 KO schizonts.Das S, Lemgruber L, Tay CL, Baum J, Meissner M. Multiple essential functions of Plasmodium falciparum actin-1 during malaria blood-stage development. BMC Biol. 2017 Aug 15;15(1):70.
See original on MMPMore information
| PlasmoDB | PF3D7_1246200 |
| GeneDB | PF3D7_1246200 |
| Malaria Metabolic Pathways | Localisation images Pathways mapped to |
| Previous ID(s) | 2277.t00443, MAL12P1.442, PFL2215w |
| Orthologs | PBANKA_1459300 , PCHAS_1461600 , PKNH_1465800 , PVP01_1463200 , PVX_101200 , PY17X_1461900 |
| Google Scholar | Search for all mentions of this gene |