PF3D7_1121600 exported protein 1 (EXP1)
Disruptability [+]
| Species | Disruptability | Reference | Submitter | |
|---|---|---|---|---|
| P. falciparum 3D7 |
Refractory |
18614010 | Theo Sanderson, Wellcome Trust Sanger Institute | |
| P. falciparum 3D7 |
Possible |
USF piggyBac screen (Insert. mut.) | USF PiggyBac Screen | |
| P. berghei ANKA |
Refractory |
RMgm-4125 | Imported from RMgmDB | |
| P. berghei ANKA |
Refractory |
RMgm-825 | Imported from RMgmDB | |
Mutant phenotypes [+]
| Species | Stage | Phenotype | Reference | Submitter |
|---|---|---|---|---|
| P. falciparum 3D7 | Asexual |
Refractory |
https://www.biorxiv.org/content/10.1101/752634v1
(Knock down)
'EXP1 knockdown was essential for intraerythrocytic development and accompanied by profound changes in vacuole ultrastructure, including increased separation of the PVM and PPM and formation of abnormal membrane structures in the enlarged vacuole lumen. [...] Intriguingly, while activity of the Plasmodium translocon of exported proteins was not impacted by depletion of EXP1, the distribution of the translocon pore-forming protein EXP2 was substantially altered.' |
Theo Sanderson, Francis Crick Institute |
| P. falciparum 3D7 | Asexual |
Refractory |
31990132 (Knock down)
'We investigated EXP1,the longest known member of this group, by adapting a CRISPR/Cpf1 genome editing system to install the TetR-DOZI-aptamers system for conditional translational control. Importantly, while EXP1 was required for intraerythrocytic development, a previously reported in vitro glutathione S-transferase activity could not account for this essential EXP1 function in vivo. EXP1 knockdown was accompanied by profound changes in vacuole ultrastructure, including apparentincreased separation of the PVM from the PPM and formation of abnormal membrane structures. Furthermore, while activity of the Plasmodium translocon of exported proteins was not impacted by depletion of EXP1, the distribution of the translocon pore-forming protein EXP2 but not the HSP101 unfoldase was substantially altered. Collectively, our results reveal a novel PVM defect that indicates a critical role for EXP1 in maintaining proper organization of EXP2 within the PVM.' |
Theo Sanderson, Francis Crick Institute |
Imaging data (from Malaria Metabolic Pathways)
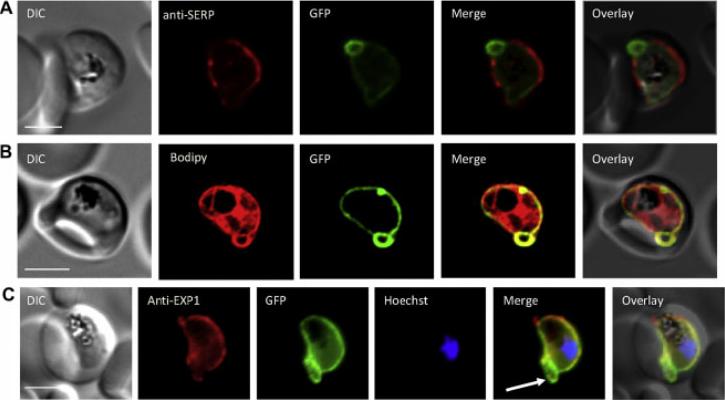
Localization of PfAK2. (A) Co-localization of PfAK2-GFP with serine-rich protein (SERA5). (B) A comparison the localization of PfAK2-GFP with Bodipy-TR-ceramide shows that the loops contain membranous material. (C) Co-localization of PfAK2-GFP with Plasmodium falciparum exported protein (Exp1) by using an anti-Exp1 antibody. Scale bar, 3 mm. PfAK2 localized to a ring-like structure around the 264 parasite, and ‘‘loops’’ apparently connected to the PVM. PfAK2-GFP co localizes with the PV resident protein SERA5 which seems to be excluded from the ‘‘loops’’ suggesting that PfAK2-GFP in actual fact associates with the membrane of the PV, rather than being found in a soluble state in the PV lumen.Ma J, Rahlfs S, Jortzik E, Heiner Schirmer R, Przyborski J, Becker K. Subcellular localization of adenylate kinases in Plasmodium falciparum. FEBS Lett. 2012 Jul 17. [Epub ahead of print]
See original on MMP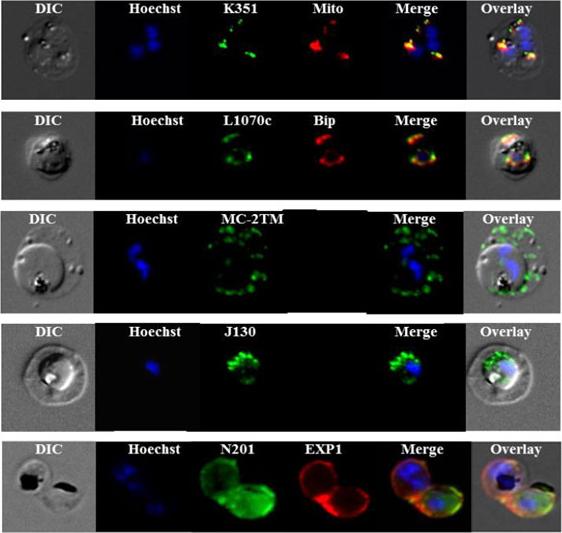
Sub-cellular localization of P. falciparum DRM-associated proteins. Immunofluorescence studies were performed on paraformaldehyde-fixed P.falciparum trophozoite-iRBCs using mouse immune sera raised against the DRM-associated proteins PFJ130, (PF10_0130) PFN201, (PF14_0201 surface protein) PFB985c, (PFB0985c) Pfmc-2TM Maurer's cleft two transmembrane protein; PFL1070c, endoplasmin homolog precursor) PFK351 (PF11_0351) HSP70. Immune sera specific for PfBip and PfEXP1-were used for co-labeling, as indicated. Mitochondria labeling was performed with MitoTracker® probe. Parasite nuclei were stained with Hoechst 33342. Microscopic observations were made on an apotome-equipped Axio Imager 2 (Carl Ziess, Jena) with a 100× apochromat objective and DIC. The differential interference contrast (DIC), merged and overlay images are presented. Bar size: 5 µm. PfK351overlays mitotracker thus localized to parasite mitochondrion. PfL1070 colocalizes with the ER-resident marker PfBiP. PfK55, a conserved hypothetical protein with unknown function, contains a SP and a ER retention signal (KDEL), but due to poor IFA, the exact subcellular localization of this protein remained elusive.Yam XY, Birago C, Fratini F, Di Girolamo F, Raggi C, Sargiacomo M, Bachi A, Berry L, Fall G, Curra C, Pizzi E, Braun-Breton C, Ponzi M. Proteomic analysis of detergent-resistant membrane microdomains in trophozoite blood stage of the human malaria parasite Plasmodium falciparum. Mol Cell Proteomics. 2013 12(12):3948-61
See original on MMP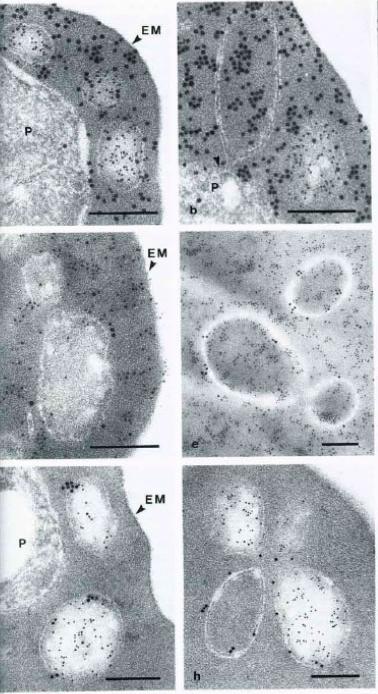
Immunoelectron micrographs of P. falciparum-infected erythrocytes showing double labeling results using anti-parasite and anti-host cell antibodies reacted with colloidal gold IgG of different particle sizes. A-B Abs directed against S-antigen (10 nm) and erythrocyte material (15 nm); D-E Abs against Exp-1 (15 nm) abd erythrocyte material (10 nm); G-H Abs against Exp-1 (15 nm) and against S-antigen (10 nm). EM – erythrocyte membrane; P – parasite; Arrowheads indicate pvm. Multiple species of vesicles, each with specifically packaged contents, are consistent with a sorting function of vesicular structures in the Plasmodium infected erythrocyte. During schizogony, two parasite antigens, an S-antigen and a parasitophorous vacuole membrane antigen, EXP-1, become packaged into such vesicles and are transported into the erythrocyte cytoplasm. At this stage of parasite development, host cell material is taken in through the parasitophorous vacuole membrane into the vacuolar space surrounding the parasite.Stenzel DJ, Kara UA. Sorting of malarial antigens into vesicular compartments within the host cell cytoplasm as demonstrated by immunoelectron microscopy. Eur J Cell Biol. 1989 49:311-8. Copyright Elsevier 2010.
See original on MMP
Intracellular localisation of PfTKL2 by immunofluorescence assay of wild type 3D7 parasites. (a) Co-localisation of PfTKL2 and the parasitophorous vacuole protein Exp1. (b) Co-localisation of PfTKL2 and the red blood cell cytoskeleton protein spectrin. DAPI was used to stain the parasite DNA. Scale bar is 2 μm. Co-localisation experiment using a parasitophorous vacuole marker (Exp1) clearly shows that a fraction of the PfTKL2 protein is exported into the RBC cytoplasm (a). Further, the co-localisation of PfTKL2 with RBC spectrin shows that PfTKL2 is present in the RBC cytosol.Abdi AI, Carvalho TG, Wilkes JM, Doerig C. A secreted Plasmodium falciparum kinase reveals a signature motif for classification of tyrosine kinase-like kinases. Microbiology. 2013 159(Pt 12):2533-47
See original on MMP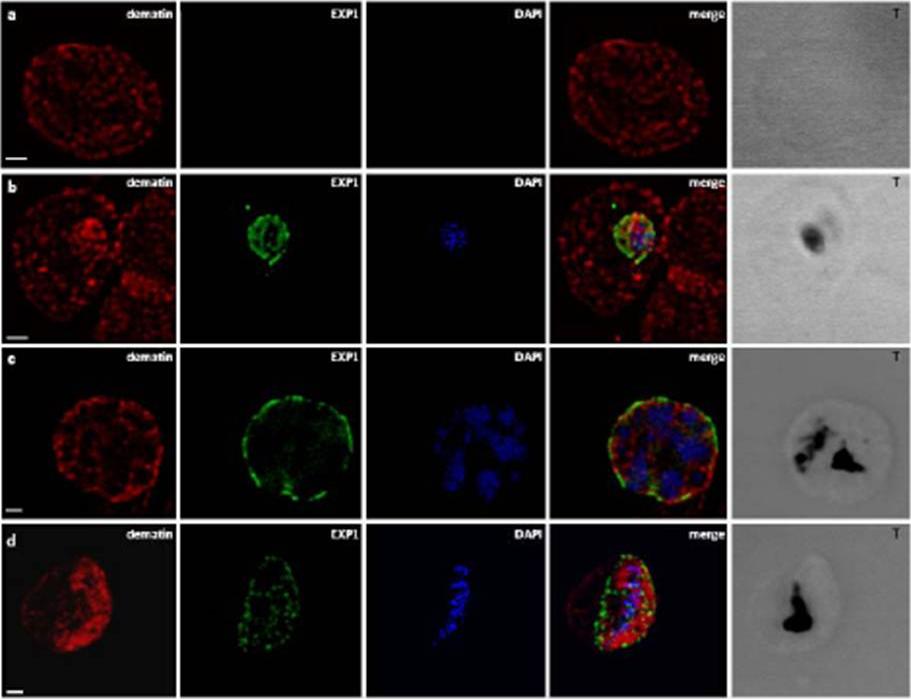
Localization of dematin in P. falciparum iRBC. B) CLSM observations of normal human RBCs (a) and erythrocytes infected by P. falciparum at different developmental stages: trophozoite (b), schizont (c), gametocyte (d), stained with the a-EXP1 serum (green), which decorate the PVM, and a-dematin md52k serum (red). Dematin is an RBC membrane associated protein. Nuclei were stained with DAPI (blue). Displayed micrographs correspond to a single stack encompassing the center of the nucleus. T, transmission light acquisition. Scale bars, 1 mm. Lalle M, Curra C, Ciccarone F, Pace T, Cecchetti S, Fantozzi L, Ay B, Braun Breton C, Ponzi M. Dematin, a component of the erythrocyte membrane-skeleton, is internalized by the malaria parasite and associates with Plasmodium 14-3-3. J Biol Chem. 2010. 286:1227-36
See original on MMP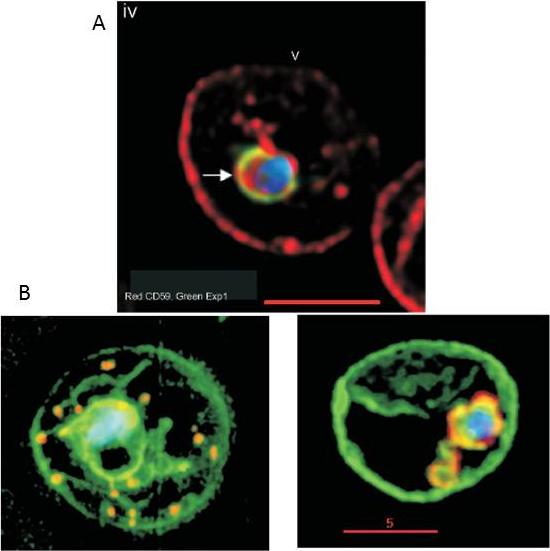
A. Internalization of host detergent-resistant membrane proteins in infected erythrocytes. Infected erythrocytes were probed with primary and relevant secondary antibodies to detect the indicated markers. CD59 (host cell marker), red; EXP1, green; . anti-CD59 antibody was directly conjugated to fluorescein isothiocyanate. The nucleus (blue) is stained with Hoechst, v indicates the periphery of the erythrocyte, arrows indicate vacuolar parasite, asterisks indicate tubular and vesicular membrane, and the scale bar is 5 mm.B. Maurer’s clefts and intraerythrocytic loops are protein domains of the tubovesicular network. (Left) Infected cells labelled with BODIPY-ceramide (green) and probed with antibody Maurer’s cleft protein (red). Blue: parasite nucleus (Hoechst). (Right) Infected cells labelled with BODIPY-ceramide and probed with antibody to PfExp1 that detects intraerythrocytic loops (red). Blue: parasite nucleus (Hoechst).Haldar K, Samuel BU, Mohandas N, Harrison T, Hiller NL. Transport mechanisms in Plasmodium-infected erythrocytes: lipid rafts and a tubovesicular network. Int J Parasitol. 2001 31:1393-401. Copyright Elsevier
See original on MMP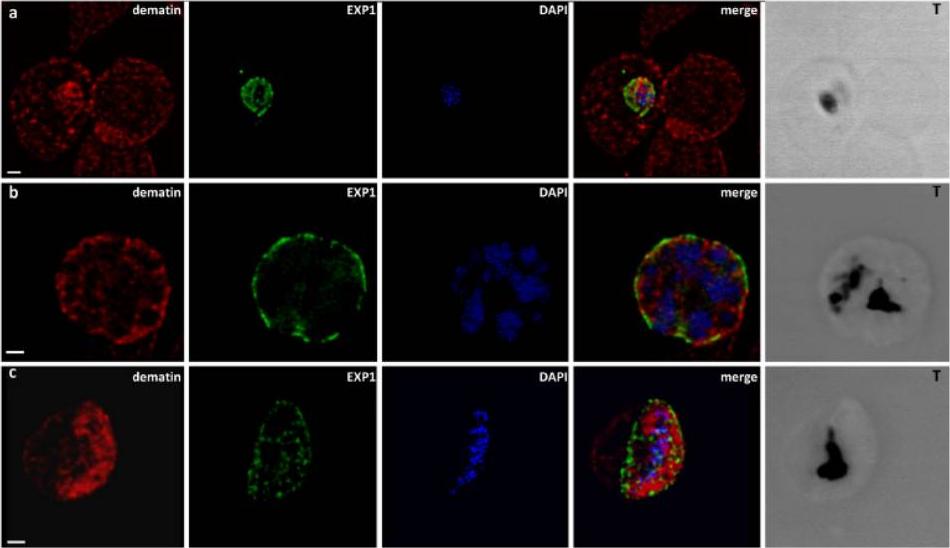
Confocal laser scanning microscopy observations of normal human RBCs (panel a) and erythrocytes infected by P. falciparum at different developmental stages: trophozoite (panel a), schizont (panel b), and gametocyte (panel c), stained with the a-EXP1 serum (green), which decorates the PVM, and the a-dematin md52k serum (red). Nuclei were stained with DAPI (blue). Displayed micrographs correspond to a single stack encompassing the center of the nucleus. T, transmission light acquisition. Scale bars, 1 mm. Dematin is an actin-binding protein of the erythrocyte membrane skeletonLalle M, Currà C, Ciccarone F, Pace T, Cecchetti S, Fantozzi L, Ay B, Breton CB, Ponzi M. Dematin, a component of the erythrocyte membrane skeleton, is internalized by the malaria parasite and associates with Plasmodium 14-3-3. J Biol Chem. 2011 286(2):1227-36.
See original on MMP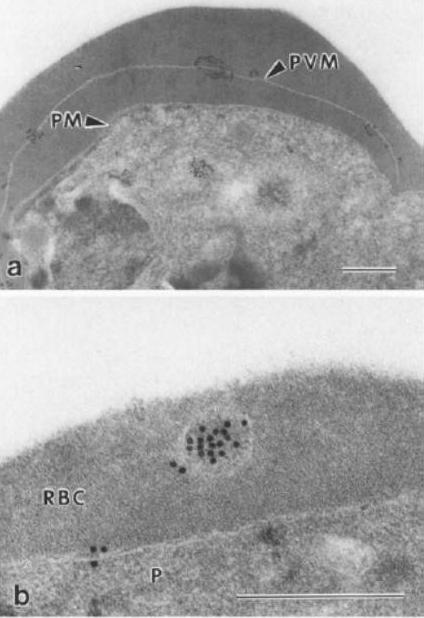
Immunoelectron micrograph showing localisation of parasitophorous vacuole membrane (PVM)-associated antigen, QFll6. Prior to embedding in LR White resin, parasitised erythrocytes were fixed with 0.05% glutaraldehyde (colloidal gold 15 nm). a Labelling is clearly associated with the pvm, which is distinct from the parasitic plasma membrane, b Antigen QF116 is also evident, clustered in vesicles within the erythrocytic cytoplasm. P, parasite; PM, parasitic membrane; P VM, parasitophorous vacuole membrane; RBC, erythrocytic cytoplasm. Bar=0.5 mmIngram LT, Stenzel DJ, Kara UA, Bushell GR. Localisation of internal antigens of Plasmodium falciparum using monoclonal antibodies and colloidal gold. Parasitol Res. 1988;74:208-15.
See original on MMP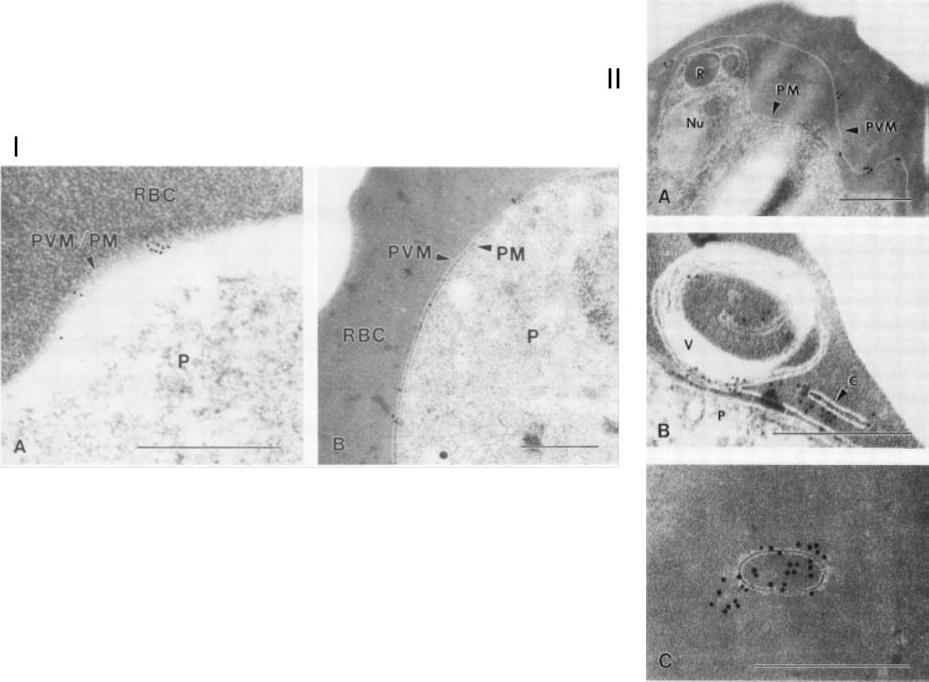
I. Immunoelectron micropgraph of P. falciparum-infected erythrocytes (FCQ-7 strain) labeled with MAb 8E7/55 and colloidal gold IgG complex. A. Schizont-infected erythrocyte showing a distinct separation of PVM from PM, with antibody labeling restricted to the vacuole membrane. Abbreviations: R, rhoptry; Nu, nucleus. (B) Membrane-bound cleft and multimembranous vesicle (V) showing antibody labeling. The parasite (P) is also shown. (C) Dense antibody labeling associated with a membrane-bound vesicle free of electron-dense material. Bars, 0.5 mm.II. Immunoelectron micropgraph of P. falciparum-infected erythrocytes (FCQ-7 strain) labeled with MAb 8E7/55 and colloidal gold IgG complex. A. Ring-stage parasite surface labeled with gold particles (diameter, 15 nm). (B) Trophozoite-stage arasite labeled with gold particles (diameter, 10 nm). Bars, 0.5 mm. Erythrocyte (RBC) and parasite (P) are separated by both the PVM and the PM.Kara UA, Stenzel DJ, Ingram LT, Bushell GR, Lopez JA, Kidson C. Inhibitory monoclonal antibody against a (myristylated) small-molecular-weight antigen from Plasmodium falciparum associated with the parasitophorous vacuole membrane. Infect Immun. 1988 Apr;56:903-9.
See original on MMP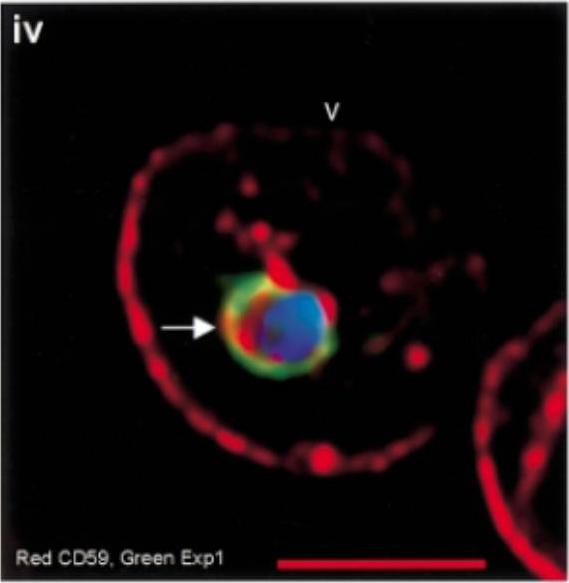
Internalization of host DRM proteins in infected erythrocytes. Infected red cells were probed with primary and relevant secondary antibodies. PfEXP1, green CD59, red. Nucleus (blue) is stained with Hoechst, v indicates the periphery of the red cell, arrows indicate vacuolar parasite, and the scale bar is 5 µm.Lauer S, VanWye J, Harrison T, McManus H, Samuel BU, Hiller NL, Mohandas N, Haldar K. Vacuolar uptake of host components, and a role for cholesterol and sphingomyelin in malarial infection. EMBO J. 2000 19:3556-64. '
See original on MMP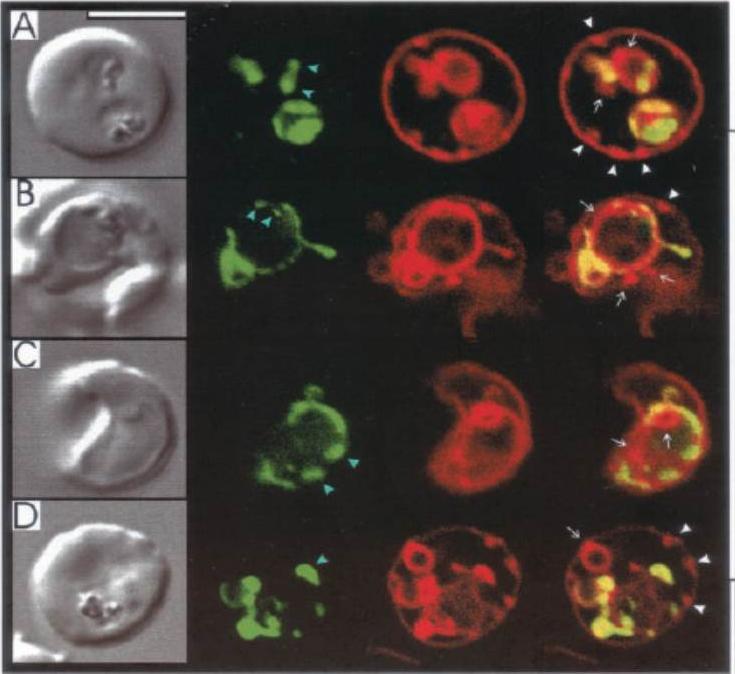
Labeling of Exp1-(1–35)-GFP transfectants with BODIPY-TR-ceramide. A–D, the confocal images in each set (left to right) represent DIC, GFP fluorescence, BODIPY-TR fluorescence, and an overlay of the GFP (green) and BODIPY-TR (red) images.Adisa A, Rug M, Klonis N, Foley M, Cowman AF, Tilley L. The signal sequence of exported protein-1 directs the green fluorescent protein to the parasitophorous vacuole of transfected malaria parasites. J Biol Chem. 2003 278:6532-42.
See original on MMP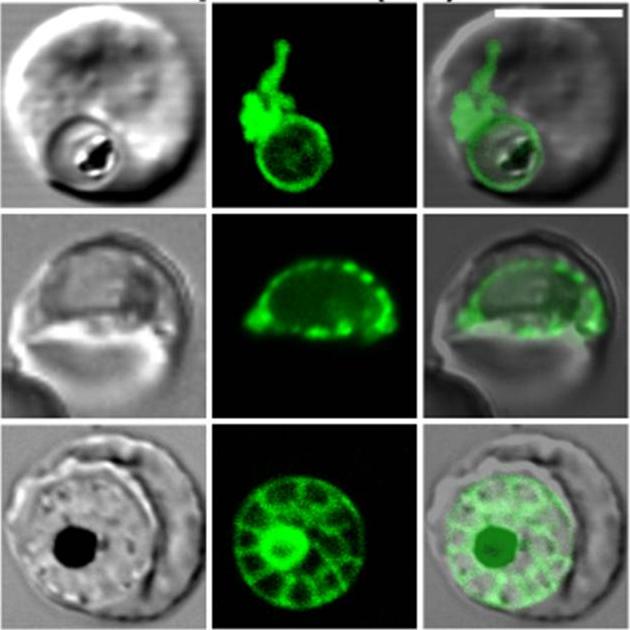
Confocal fluorescence microscopy images of transfected 3D7 P. falciparum-infected RBCs expressing a GFP chimera of a fragment of Exp-1 that is directed to the parasitophorous vacuole. DIC image, the GFP fluorescence signal and an overlay of Exported protein-11-35-GFP transfectant. Scale bar = 5 µm.Top and middle rows show trophozoite stage parasite. The top cell shows extensions of the parasitophorous vacuole to form the tubulovesicular network (TVN). The middle cell shows a necklace of beads pattern in parasitophorous vacuole. In the schizont stage parasite (bottom row) the protein accumulates around the individual merozoites forming a segmented/ wagon wheel pattern around a fluorescent residual body. Tilley L, McFadden G, Cowman A, Klonis N. Illuminating Plasmodium falciparum-infected red blood cells. Trends Parasitol. 2007 23:268-77. Copyright Elsevier
See original on MMP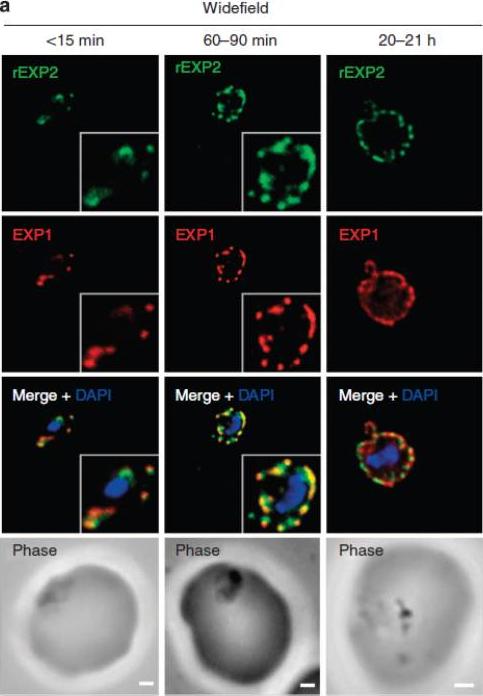
EXP1 localizes to PVM domains with PTEX. Parasites were fixed at <15 min, 60–90 min and 20–21 h after invasion and labelled by IFA for EXP1 (red), EXP2 (green) and the nucleus (40,6–diamidino-2-phenylindole (DAPI), blue). (a) Imaging of all samples by widefield deconvolution microscopy (scale bar, 1 mm). when EXP2 localization was compared with that of a DRM-enriched protein, the exported protein 1 (EXP1), clear co-incidence of fluorescence was seen using widefield deconvolution microscopy of parasites fixed <15 min, 60–90 min and 20-21 h post invasion, although EXP1 appeared to become more widely distributed around the PVM in the latter sample.Riglar DT, Rogers KL, Hanssen E, Turnbull L, Bullen HE, Charnaud SC, Przyborski J, Gilson PR, Whitchurch CB, Crabb BS, Baum J, Cowman AF. Spatial association with PTEX complexes defines regions for effector export into Plasmodium falciparum-infected erythrocytes. Nat Commun. 2013 Jan 29;4:1415.
See original on MMP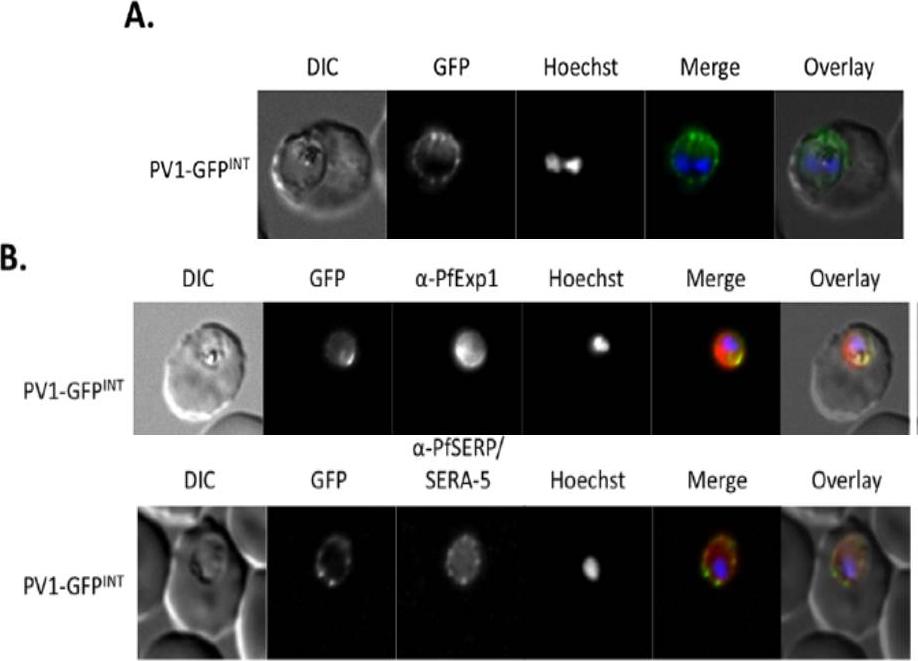
Live cell imaging. (A) Left: live cell imaging of the PV1-GFPINT transgenic line. (B) Upper panel, colocalisation of PV1-GFP chimera with PfExp1. Lower panel, colocalisation of PV1-GFP chimera with PfSERA-5. In merge and overlay: GFP, green; specific antibody, red; Hoechst, blue. Immunofluorescence co-localisation using antibodies against the PV and PVM markers SERA-5 and Exp-1 respectively verified that this fluorescent signal represented the PV.Chu T, Lingelbach K, Przyborski JM. Genetic evidence strongly support an essential role for PfPV1 in intra-erythrocytic growth of P. falciparum. PLoS One. 2011 6(3):e18396.
See original on MMP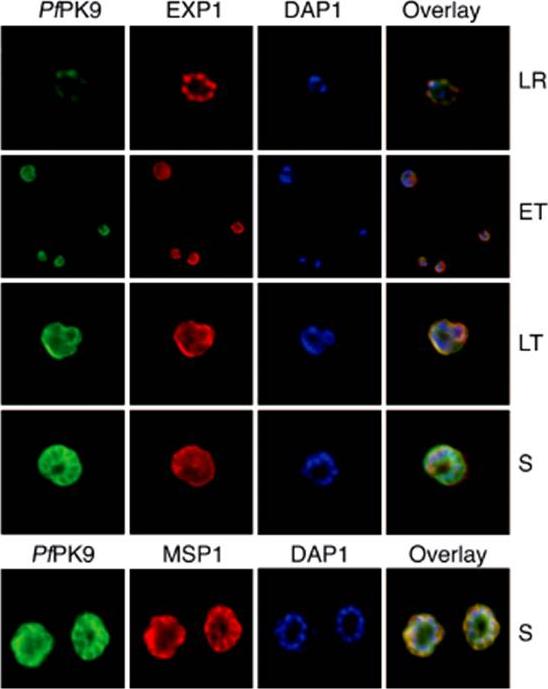
PfPK9 expression and localization across the intraerythrocytic life cycle. Immunofluorescence using polyclonal anti-PfPK9 antibody reveals a punctuate staining at late ring stage during which PfPK9 (green) appears to colocalize with exported protein 1 (red) in several spots. In addition to parasitophorous membrane staining (using EXP-1 PF11_0224 as PVM marker), diffused cytoplasmic staining is observed during both early and late trophozoites. During the schizont stage, the expression pattern emerges to be like merozoite surface protein 1 PFI1475w (red), a plasma membrane protein.Philip N, Haystead TA. Characterization of a UBC13 kinase in Plasmodium falciparum. Proc Natl Acad Sci U S A. 2007 104:7845-7850.
See original on MMP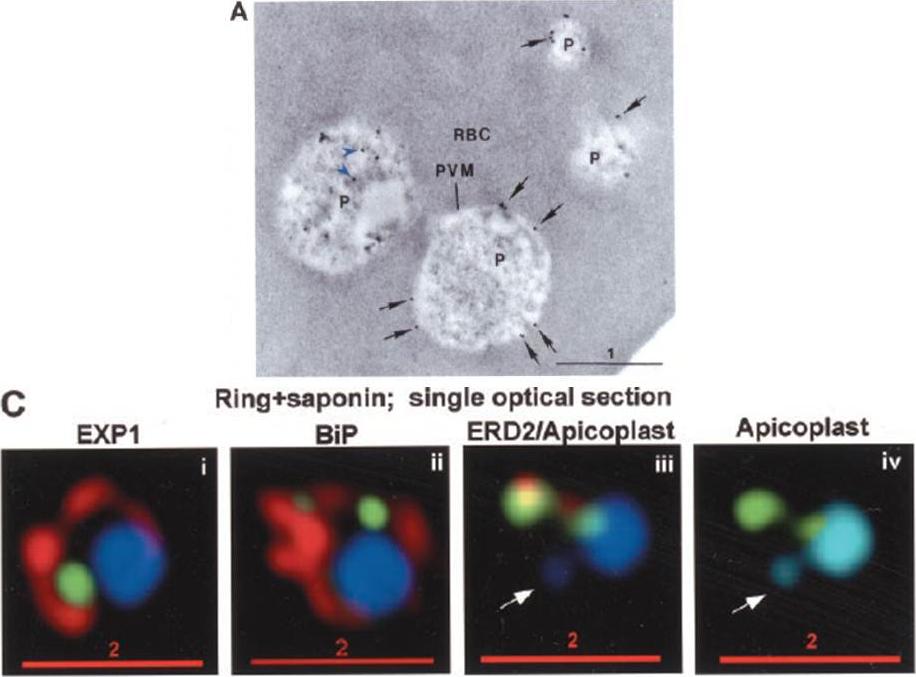
PTS-GFP is detected in the parasitophorous vacuole and in apposition with the Golgi in early rings. A, immunoelectron micrograph of rings showing localization of PTS (plastid-targeting-signal)-GFP in the parasitophorous vacuole and PVM (black arrows) as well as within the parasite (P; blue arrowheads). RBC, red blood cell. Scale bar, 1 mm.. C, i–iv: single optical sections showing green fluorescence in young rings permeabilized with 0.01% saponin relative to secretory markers PfEXP1, PfBiP, PfERD2 (shown in red in i–iii), and apicoplast DNA (marked with an arrow in iii and iv), as detected by indirect immunofluorescence and DeltaVision Microscopy. In C, iv, the Hoechst stain is pseudo-colored cyan to facilitate visualization of apicoplast DNA.Cheresh P, Harrison T, Fujioka H, Haldar K. Targeting the malarial plastid via the parasitophorous vacuole. J Biol Chem. 2002 277:16265-77.
See original on MMP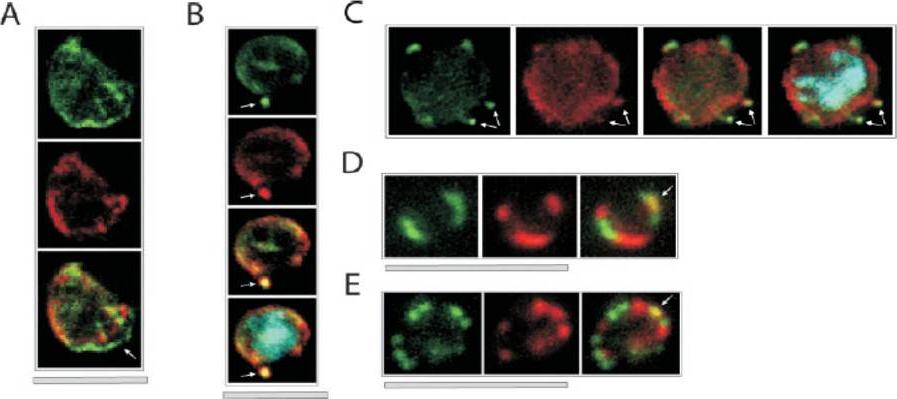
Colocalization of ETRAMPs with EXP-1 and SERP. The first image in each set shows ETRAMP labeling (green, FITC), the second Texas-Red-labeled SERP (A) or EXP-1 (B–F), and the third image represents the merged picture. The fourth panel in B and C depicts the merged picture, including nuclear staining (DAPI). (A) Confocal microscopy analysis of SERP and ETRAMP4 colocalization in schizont stage parasites (dried and 1% formaldehyde fixed). The ETRAMP4 signal seems partially detached from the PV content (arrow). (B and C) ETRAMP4 and EXP-1 localize to dots, probably representing vesicular structures that are detached from the PVM (E) or seem connected to the PVM (F). Dots are indicated by arrows. (D and E) Fluorescent microscopic analysis of ETRAMP and EXP-1 costaining of a ring-stage parasite displaying an even circular pattern (D, 0.1% formaldehyde-fixed IRBCs, ETRAMP10.1) or a beads on a string pattern (E, unfixed IRBCs, ETRAMP2). Yellow color shows regions of overlap (arrows). This indicated that ETRAMPs and EXP-1 localize to different regions of the PVM.Spielmann T, Fergusen DJ, Beck HP. etramps, a new Plasmodium falciparum gene family coding for developmentally regulated and highly charged membrane proteins located at the parasite-host cell interface. Mol Biol Cell. 2003 14:1529-44.
See original on MMP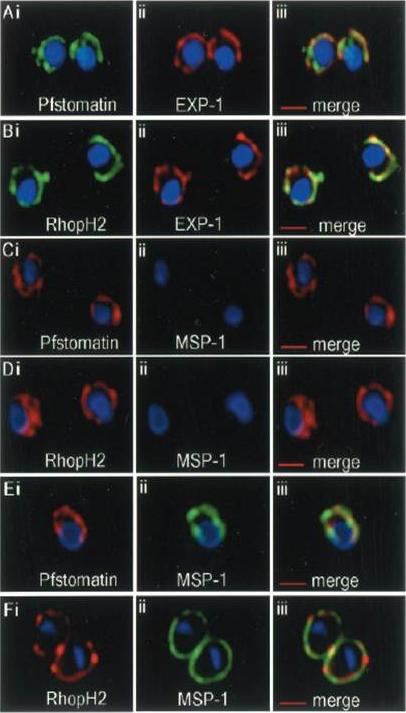
Localization of Pfstomatin and RhopH2 to the PVM in ring-infected erythrocytes (0–8 h post-invasion). Cells were subjected to a standard indirect immunofluorescence assay where samples were permeabilized with saponin and probed with antibodies to: Pfstomatin (Ai; green), RhopH2 (Bi; green), and the PVM marker EXP-1 (Aii and Bii; red); the merged images are shown in Aiii and Biii. In C and D, ring-infected erythrocytes were treated with tetanolysin and incubated with antibodies to: Pfstomatin (Ci; red), RhopH2 (Di; red) and the PPM marker MSP-1 (Cii and Dii; green); the merged images are shown in Ciii and Diii. In E and F, rings treated with tetanolysin and permeabilized with saponin were incubated with antibodies to: Pfstomatin (Ei; red), RhopH2 (Fi; red), and MSP-1 (Eii and Fii; green); the merged images are shown in Diii and Fiii. All images shown are single optical section. The nucleus (blue) was stained with Hoechst 33342. Scale bars represent 2 mm. Hiller NL, Akompong T, Morrow JS, Holder AA, Haldar K. Identification of a stomatin orthologue in vacuoles induced in human erythrocytes by malaria parasites. A role for microbial raft proteins in apicomplexan vacuole biogenesis. J Biol Chem. 2003 278:48413-21.
See original on MMP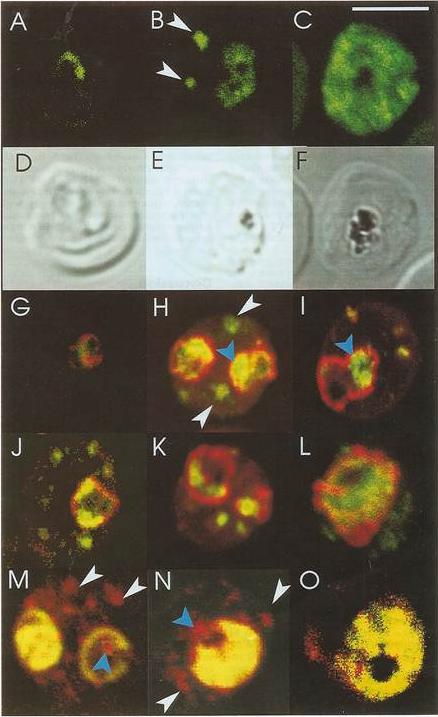
Confocal immunofluorescence of PfSar1p. Panels A-C: Parasitized erythrocyes (D10 strain) were labeled with affinity purified anti=PfSar1p antiserum followed by fluorescein-conjugated anti-rabbit antiserum (green). Panels D-F: Transmission images of panels A-C. Panels G-L: Dual labeling of affinity-purified rabbit anti-PfSar1p antiserum followed by fluorescein-conjugated anti-rabbit antiserum (green) and a murine monoclonal antibody recognizing the parasitophorous vacuole-located protein, Exp-1, followed by rhodamine-conjugated anti-mouse antiserum (red). Sar1p in the trophozoite is clearly located in the erythrocyte cytosol (G-K) and in the schizont it occupies most of it (L). Panels M-O: Dual labeling of affinity-purified rabbit anti-PfSar1p antiserum followed by rhodamine-conjugated anti-mouse antiserum (red) and a biotin-conjugated anti-PfERC antiserum followed by fluorescein-conjugated anti-rabbit antiserum (green). PfERC and PfSar1p labeling patterns do not completely coincide, indicating that PfSar1p is not confined to the ER. An optical slice was collected through the center of the parasite by confocal microscopy. Some PfSar1p-containing structures in the erythrocyte cytosol are marked with white arrowheads. Some PfSar1p-containing structures in the parasite cytosol are marked with blue arrowheads. The bar in panel C corresponds to 5 mm.Albano FR, Berman A, La Greca N, Hibbs AR, Wickham M, Foley M, Tilley L. A homologue of Sar1p localises to a novel trafficking pathway in malaria-infected erythrocytes. Eur J Cell Biol. 1999 78:453-462. Copyright Elsevier 2010.
See original on MMP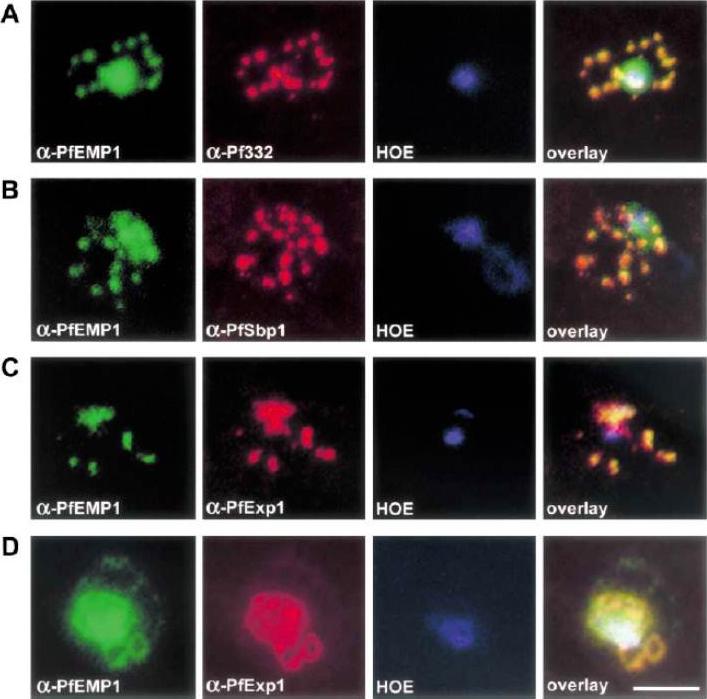
Co-localization of PfEMP1 with Pf332, PfSbp1 and PfExp-1 in P. falciparum-infected erythrocytes. Double immuno-fluorescence images of infected erythrocytes at the trophozoite stage with antibodies against: (A) PfEMP1 (a-PfEMP1) and Pf332 (a-Pf332); B) PfEMP1 (a-PfEMP1) and PfSbp1 (a-PfSbp1); (C, D) PfEMP1 (a-PfEMP1) and PfExp-1 (a-PfExp1). Different morphological appearances of the structures stained with antibodies against PfEMP1 and PfExp-1 are shown in (C and D). (HOE), DNA staining by Hoechst. (overlay), Overlay of the micrographs shown in a row. Representative examples of three independent experiments are shown. Bar, 5 mm.Wickert H, Wissing F, Andrews KT, Stich A, Krohne G, Lanzer M. Evidence for trafficking of PfEMP1 to the surface of P. falciparum-infected erythrocytes via a complex membrane network. Eur J Cell Biol. 2003 82:271-84.
See original on MMP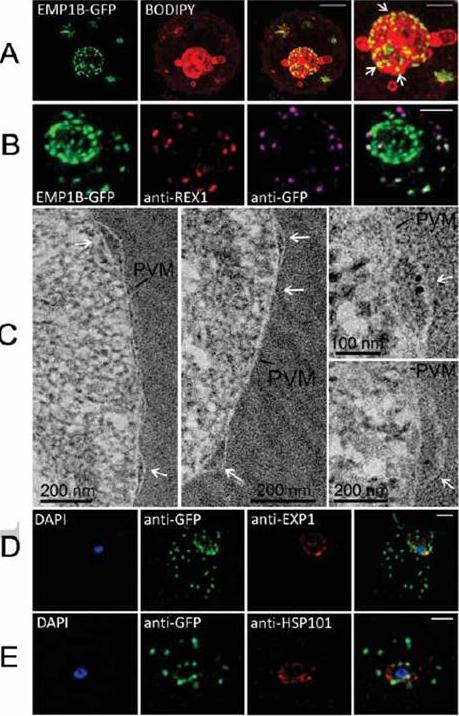
Characterization of an intermediate compartment in PfEMP1 export. (A) Transfectants (ITK strain) expressing the PfEMP1B-GFP chimera were co-labeled with BODIPY-ceramide and imaged using 3D-SIM. See higher magnification image at right. (B) PfEMP1B-GFP transfectants (endogenous GFP fluorescence; green) were permeabilized with EqtII and antibody-accessible epitopes were labeled with anti-GFP (magenta) and anti-REX1 (red). (C) PfEMP1B-GFP transfectants were fixed and resin-embedded and sections were labeled with anti-GFP and protein-A-gold (6 nm left panels, 10 nm right panels). Labeling is observed at bulges in the PV (arrows). (D) Smears of PfEMP1B-GFP transfectant-infected RBCs were fixed with acetone: methanol and stained with antibodies recognizing GFP and Exp1 (D) or Hsp101 (E). Scale bars = 2 μm (A, first three columns), 1 μm (A, last column), 2 μm (B), as marked (C) and 2 μm (D, E).McMillan PJ, Millet C, Batinovic S, Maiorca M, Hanssen E, Kenny S, Muhle RA, Melcher M, Fidock DA, Smith JD, Dixon MW, Tilley L. Spatial and temporal mapping of the PfEMP1 export pathway in Plasmodium falciparum. Cell Microbiol. 15(8):1401-18
See original on MMP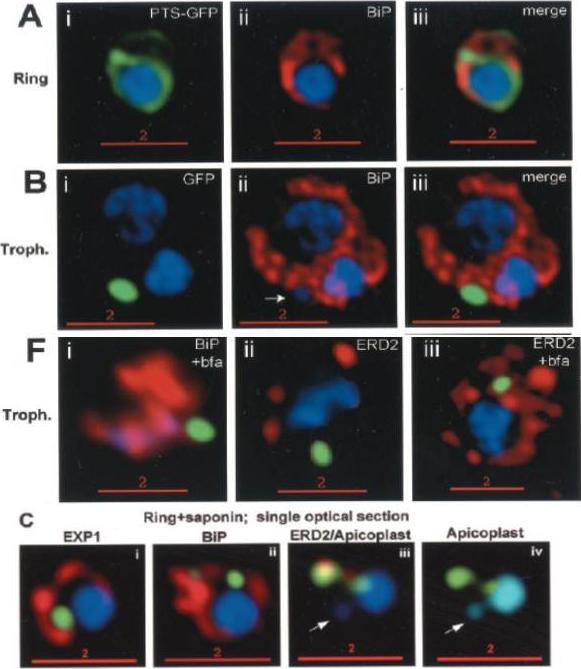
Stage-specific localization of apicoplast-targeted GFP. Distribution of green fluorescence and PfBiP (red) in a early-ring (A, i–iii) and a trophozoite (B, i–iii; a late, ~33 h, trophozoite with two nuclei stained in blue is shown). Distribution of green fluorescence and indicated secretory marker (red) in trophozoites incubated. Blue indicates DNA stained with Hoechst 33342. White arrows in B, ii, D, ii, and E, ii, indicate apicoplast DNA. Scale bars as indicated in microns. C, i–iv: single optical sections showing green fluorescence in young rings permeabilized with 0.01% saponin relative to secretory markers PfEXP1, PfBiP, PfERD2 (shown in red in i–iii), and apicoplast DNA (marked with an arrow in iii and iv), as detected by indirect. In C, iv, the Hoechst stain is pseudo-colored cyan to facilitate visualization of apicoplast DNA.Cheresh P, Harrison T, Fujioka H, Haldar K. Targeting the malarial plastid via the parasitophorous vacuole. J Biol Chem. 2002 277:16265-77.
See original on MMP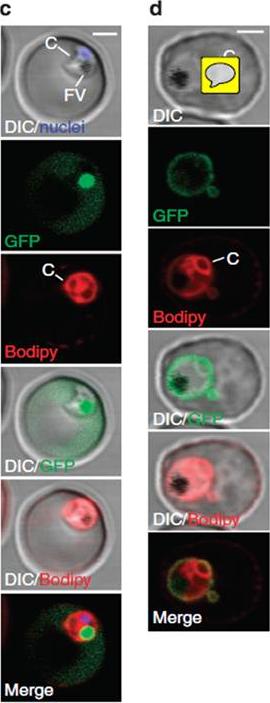
(c) A parasite of a line exporting GFP into the host cell and stained with Bodipy-TR-C5-ceramide (red) indicates that the cavity contains host cell cytosol. A single confocal z-section is shown. Note that the nucleus (blue) and the FV (filled with reinternalized GFP) are clearly distinct from the cavity. (d) A cell line exporting GFP into the PV shows a fluorescence pattern similar to Bodipy-TR-C5-ceramide staining (red). A single confocal section is shown. Parasites expressing GFP in the host cell cytosol (using the REX3 export motif) showed fluorescence in the cavity (c), whereas a cell line expressing GFP in the PV (using the EXP1 signal peptide) showed staining similar to Bodipy-TR-C5-ceramide (d), indicating that the cavity represents an invagination of the PVM and the parasite plasma membrane, forming a space filled with host cell cytosol.Grüring C, Heiber A, Kruse F, Ungefehr J, Gilberger TW, Spielmann T. Development and host cell modifications of Plasmodium falciparum blood stages in four dimensions. Nat Commun. 2011 2:165.
See original on MMP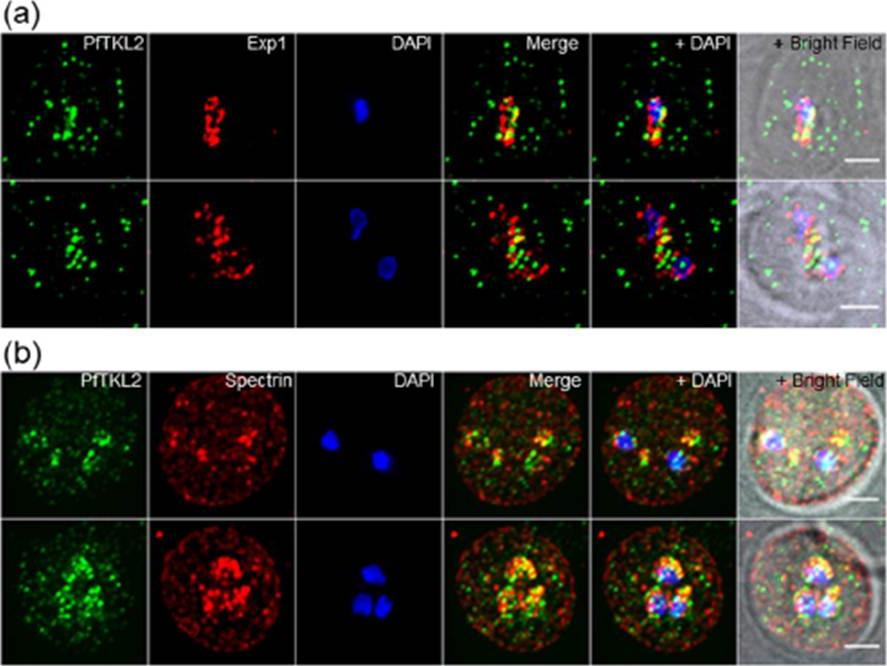
Intracellular localisation of PfTKL2 by immunofluorescence assay of wild type 3D7 parasites. (a) Co-localisation of PfTKL2 and the parasitophorous vacuole protein Exp1. (b) Co-localisation of PfTKL2 and the red blood cell cytoskeleton protein spectrin. DAPI was used to stain the parasite DNA. Scale bar is 2 μm. Co-localisation experiment using a parasitophorous vacuole marker (Exp1) clearly shows that a fraction of the PfTKL2 protein is exported into the RBC cytoplasm (a). Further, the co-localisation of PfTKL2 with RBC spectrin shows that PfTKL2 is present in the RBC cytosol.Abdi AI, Carvalho TG, Wilkes JM, Doerig C. A secreted Plasmodium falciparum kinase reveals a signature motif for classification of tyrosine kinase-like kinases. Microbiology. 2013 159(Pt 12):2533-47
See original on MMP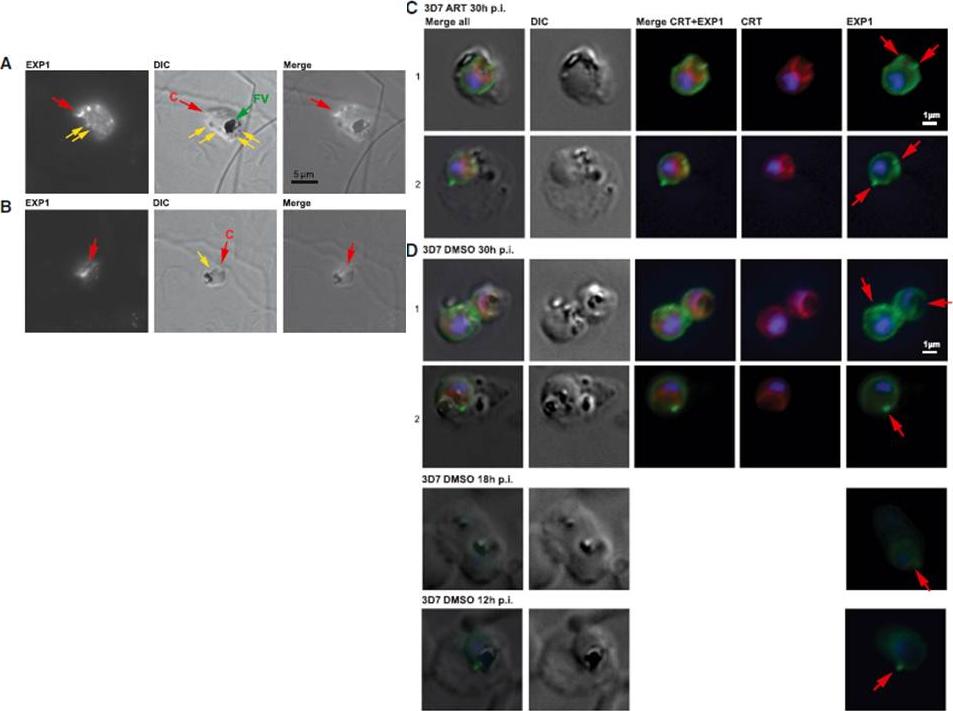
(A) Immunofluorescence microscopy of 3D7 trophozoite parasites labeled with anti-EXP1 antibodies (EXP1) shows evidence of peripheral expression of EXP1 that colocalizes with an apparent cytostomic invagination (C, red arrow; DIC panel). A fully formed trophozoite stage food vacuole (FV, green arrow) presents a large accumulation of hemozoin, whereas small darker spots (yellow arrows) might be vesicles that have pinched off from the invagination. (B) A late ring stage parasite displays a prominent spherical invagination that also partly corresponds to a convexly shaped EXP1 expression signal; small vesicles (yellow arrow) may become components of an internal pre-FV compartment (dark irregular structure). (C) Trophozoite stage 3D7 parasites exposed to ART indicate peripheral EXP1 expression (row C1) or enhanced expression foci at the PVM (row C2). (D) Control trophozoites (DMSO) displayed similar morphology in EXP1 expression patterns to the drug-exposed parasites. Antibodies to the FV marker CRT (P. falciparum chloroquine resistance marker) indicate that EXP1 expression was largely independent of FV location.Lisewski AM, Quiros JP, Ng CL, Adikesavan AK, Miura K, Putluri N, Eastman RT, Scanfeld D, Regenbogen SJ, ltenhofen L, Llinás M, Sreekumar A, Long C, Fidock DA, Lichtarge O. Supergenomic Network Compression and the Discovery of EXP1 as a Glutathione Transferase Inhibited by Artesunate. Cell. 2014 158(4):916-28.
See original on MMP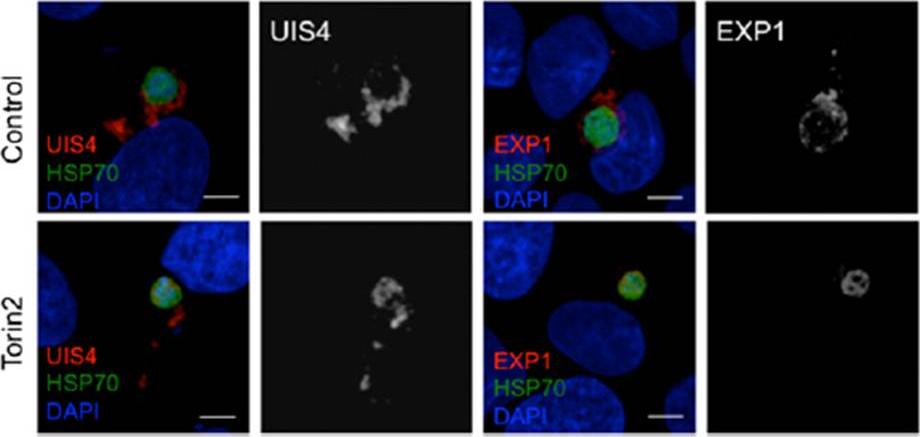
Effect of Torin2 treatment on the PVM and UIS4 localization in young liver stage trophozoites. (C) Effect of Torin2 treatment on UIS4 and EXP1 during early liver stage schizogony. UIS4 and EXP1 were localized almost completely outside of the parasite soma in control. Torin2 treatment led to a dramatic mislocalization of UIS4 and EXP1, with signal largely found in the parasite soma.Hanson KK, Ressurreição AS, Buchholz K, Prudêncio M, Herman-Ornelas JD, Rebelo M, Beatty WL, Wirth DF, Hänscheid T, Moreira R, Marti M, Mota MM. Torins are potent antimalarials that block replenishment of Plasmodium liver stage parasitophorous vacuole membrane proteins. Proc Natl Acad Sci U S A. 2013 110(30):E2838-47.
See original on MMP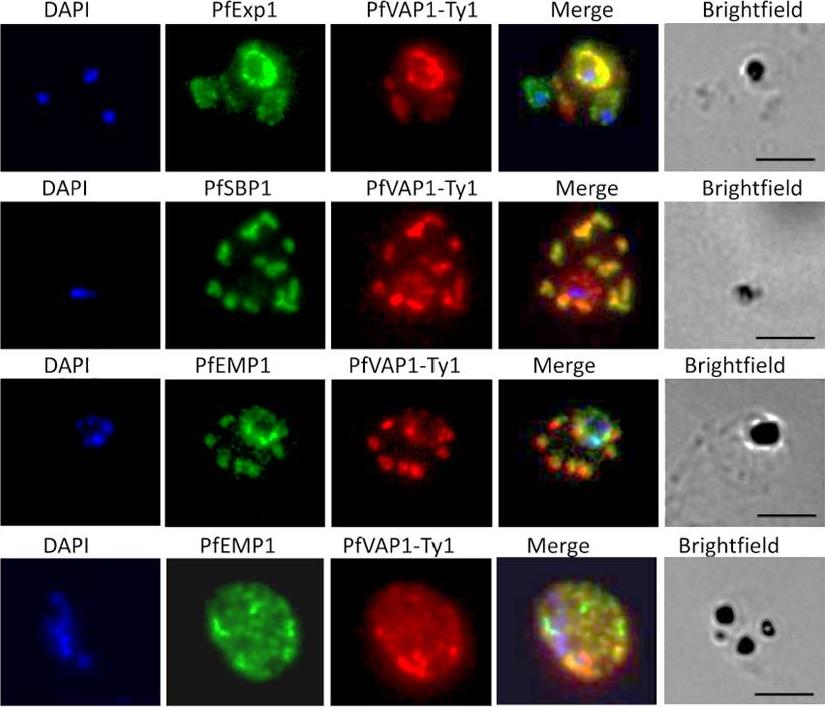
Localisation of PfVAP1 in complemented D10 parasites. Ty1-tagged D10 Pfvap1 parasites were labelled with anti-Ty1 monoclonal antibody to determine the localisation of the protein. In early trophozoites PfVAP1 is associated with the parasitophorous vacuole (PV) as determined by double labelling with PfEXP1. It is then trafficked via the Maurer’s clefts (PfSBP1) to the infected erythrocyte membrane. In the Maurer’s clefts, PfVAP1 co-localises with PfEMP1 (3rd row) and appears to localise in distinct regions, separate from PfEMP1 at the erythrocyte membrane (4th row). Scale bars = 5 μm.Nacer A, Claes A, Roberts A, Scheidig-Benatar C, Sakamoto H, Ghorbal M, Lopez-Rubio JJ, Mattei D. Discovery of a novel and conserved Plasmodium falciparum exported protein that is important for adhesion of PfEMP1 at the surface of infected erythrocytes. Cell Microbiol. 2015 Feb 23
See original on MMP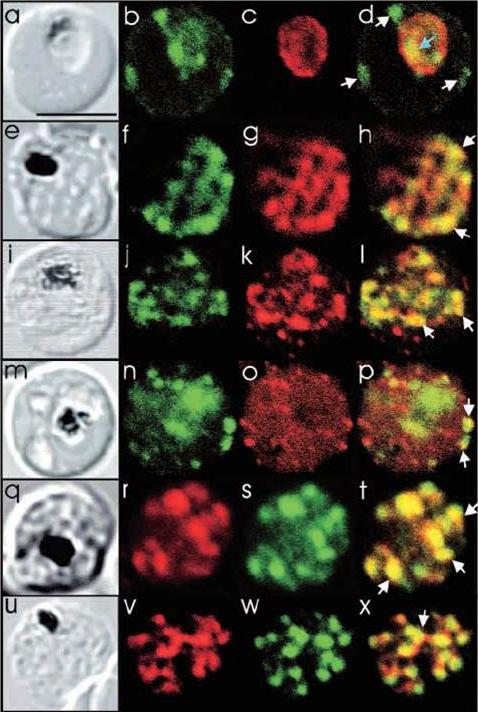
Comparison of the location of PfSec31p with that of other parasite antigens in P. falciparum-infected erythrocytes. (a-d) Asynchronous infected erythrocytes (K1 strain) were probed with affinity-purified rabbit anti-PfSec31(WD) antiserum followed by a fluorescein-conjugated anti-rabbit IgG (b; green fluorescence) and a murine monoclonal antibody recognising the PV-located protein Exp1 followed by rhodamine conjugated anti-mouse IgG (c; red fluorescence). An optical slice was collected through the centre of the parasite by confocal microscopy. Some PfSec31p-containing structures in the erythrocyte cytosol are marked with white arrowheads and a PfSec31p-containing structure in the parasite cytosol is marked with a blue arrowhead (d). (a) A transmission image of (b-d). (e-l) Asynchronous infected erythrocytes (K1 strain) were probed with mouse anti-PfSec31 (WD) antiserum followed by a fluorescein-conjugated anti-mouse IgG (green fluorescence) and a rabbit anti-PfSar1p antiserum followed by Alexa-Fluor-568-conjugated anti-rabbit IgG (red fluorescence). Optical slices were collected near the surface of the parasitised erythrocytes. Some PfSec31p- and PfSar1p-containing structures are marked with arrowheads. (m-p) Asynchronous infected erythrocytes were probed with affinity-purified rabbit anti-PfSec31( WD) antiserum followed by fluorescein-conjugated anti-rabbit IgG (green fluorescence) and a murine monoclonal antibody recognising PfEMP3, followed by Alexa-Fluor-568-conjugated anti-mouse IgG (red florescence). An optical slice was collected near the surface of the parasitised erythrocyte. Two structures in which there is partial overlap of PfSec31p and PfEMP3 in the erythrocyte cytosol are marked with arrowheads. (q-x) Asynchronous infected erythrocytes were probed with a mouse anti-PfSec31(WD) antiserum followed by Alexa-Fluor-568-conjugated anti-mouse IgG (red fluorescence) and an affinity-purified rabbit anti-PfEMP1 antibody followed by fluorescein-conjugated anti-rabbit IgG (green florescence). Optical slices were collected near the surface of the parasitised erythrocytes. Some PfSec31p- and PfEMP1-containing structures in the erythrocyte cytosol are marked with arrowheads. Bar in (a), 5 mm.Adisa A, Albano FR, Reeder J, Foley M, Tilley L. Evidence for a role for a Plasmodium falciparum homologue of Sec31p in the export of proteins to the surface of malaria parasite-infected erythrocytes. J Cell Sci. 2001 114(Pt 18):3377-86.
See original on MMP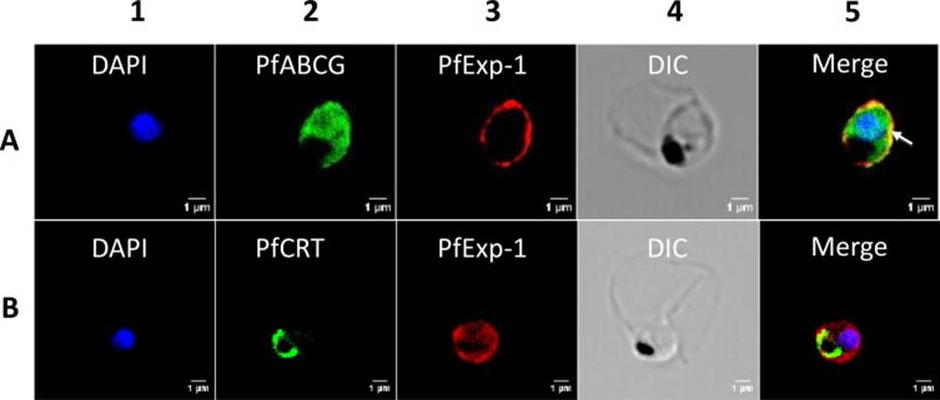
Co-localization of PfExp-1 and PfABCG in P. falciparum-infected erythrocytes. Double immuno-histochemical staining of infected erythrocytes at trophozoites stage with antibodies against PfABCG and PfExp-1 (Panel A) and PfCRT and PfExp-1 (Panel B). Column 1 shows the nuclear DNA staining with DAPI (blue). In columns 2 and 3, PfABCG signal (green) (A) and PfCRT signal (green) (B) are resolved with Alexa-fluor 488-labeled goat anti-rabbit secondary antibody, and PfExp-1 signal (red) (A and B) is resolved with Alexa-fluor 594-labeled goat anti-rabbit secondary antibody. Columns 4 and 5 show respectively the differential interference contrast (DIC) of infected erythrocyte and the merger of column 1, 2 and 3 without DIC. The digestive vacuole membrane staining of PfCRT does not overlap with PfExp-1 staining while the plasma membrane staining of PfABCG partially overlaps with the parasitophorous vacuole membrane signal of PfExp-1 as indicated by the arrow.Edaye S, Georges E. Characterization of native PfABCG protein in Plasmodium falciparum. Biochem Pharmacol. 2015 Jul 31. [Epub ahead of print] PMID:
See original on MMP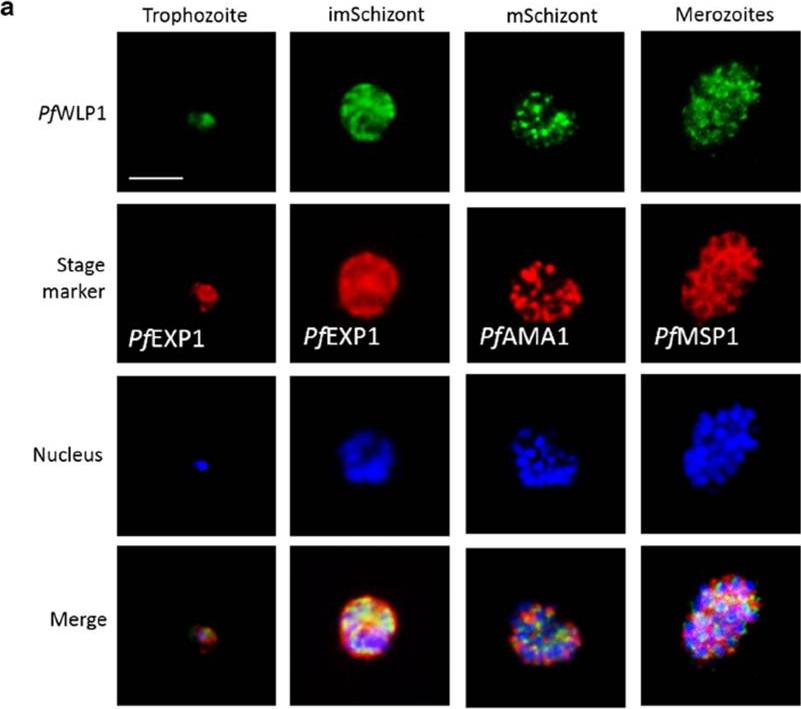
PfWLP1 is expressed in the asexual blood stages of Plasmodium falciparum. a Expression of PfWLP1 in the asexual blood stages. PfWLP1 was immunolabelled with anti-PfWLP1-rp2 antisera (green); the asexual blood stages were visualized with antisera against PfEXP1, PfAMA1 and PfMSP1 (red). The parasite nuclei were highlighted by Hoechst nuclear stain (blue). ImSchizont, immature schizont; mSchizont, mature schizont. Bar 5 μm. Data are representative of five independent experiments each.von Bohl A, Kuehn A, Simon N, Ngongang VN, Spehr M, Baumeister S, Przyborski JM, Fischer R, Pradel G. A WD40-repeat protein unique to malaria parasites associates with adhesion protein complexes and is crucial for blood stageprogeny. Malar J. 2015 Nov 4;14(1):435.
See original on MMPMore information
| PlasmoDB | PF3D7_1121600 |
| GeneDB | PF3D7_1121600 |
| Malaria Metabolic Pathways | Localisation images Pathways mapped to |
| Previous ID(s) | PF11_0224 |
| Orthologs | PBANKA_0926700 , PCHAS_0917600 , PKNH_0919300 , PVP01_0922200 , PVX_091700 , PY17X_0928700 |
| Google Scholar | Search for all mentions of this gene |