PF3D7_0702400 small exported membrane protein 1 (SEMP1)
Disruptability [+]
| Species | Disruptability | Reference | Submitter |
|---|---|---|---|
| P. falciparum 3D7 |
Possible |
USF piggyBac screen (Insert. mut.) | USF PiggyBac Screen |
| P. falciparum 3D7 |
Possible |
25062022 Knock-out was done in 3D7 parasites using single crossover gene disruption. No difference to PfEMP1 export under culture conditions. No effect seen on asexual parasite growth and parasites were able to progress normally to stage V gametocytes similary to that of wild type parasites. |
Thorey Jonsdottir, Burnet Institute |
Mutant phenotypes [+]
| Species | Stage | Phenotype | Reference | Submitter |
|---|---|---|---|---|
| P. falciparum 3D7 | Asexual |
No difference |
25062022 Knock-out was done in 3D7 parasites using single crossover gene disruption. No difference to PfEMP1 export under culture conditions. No effect seen on asexual parasite growth and parasites were able to progress normally to stage V gametocytes similary to that of wild type parasites. |
Thorey Jonsdottir, Burnet Institute |
| P. falciparum 3D7 | Gametocyte |
No difference |
25062022 Knock-out was done in 3D7 parasites using single crossover gene disruption. No difference to PfEMP1 export under culture conditions. No effect seen on asexual parasite growth and parasites were able to progress normally to stage V gametocytes similary to that of wild type parasites. |
Thorey Jonsdottir, Burnet Institute |
Imaging data (from Malaria Metabolic Pathways)
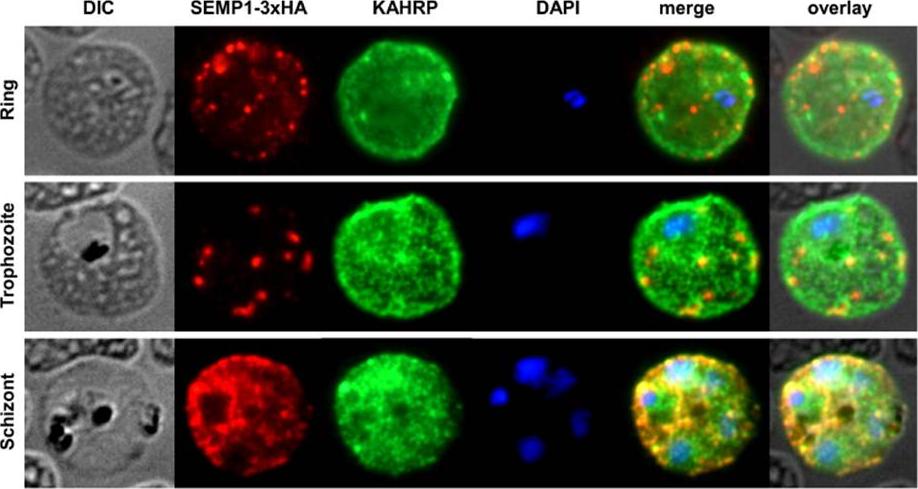
SEMP1 expression during blood stage development. Immunofluorescence assays of MeOH-fixed RBCs infected with 3D7 expressing SEMP1 with a C-terminal 3xHA tag, co-labelled with rat a-HA and mouse a-KAHRP antibodies. In later stages SEMP1-3xHA is further transported to the RBC membrane and partially localizes with KAHRP.Dietz O, Rusch S, Brand F, Mundwiler-Pachlatko E, Gaida A, Voss T, Beck HP. Characterization of the Small Exported Plasmodium falciparum Membrane Protein SEMP1. PLoS One. 2014 9(7):e103272.
See original on MMP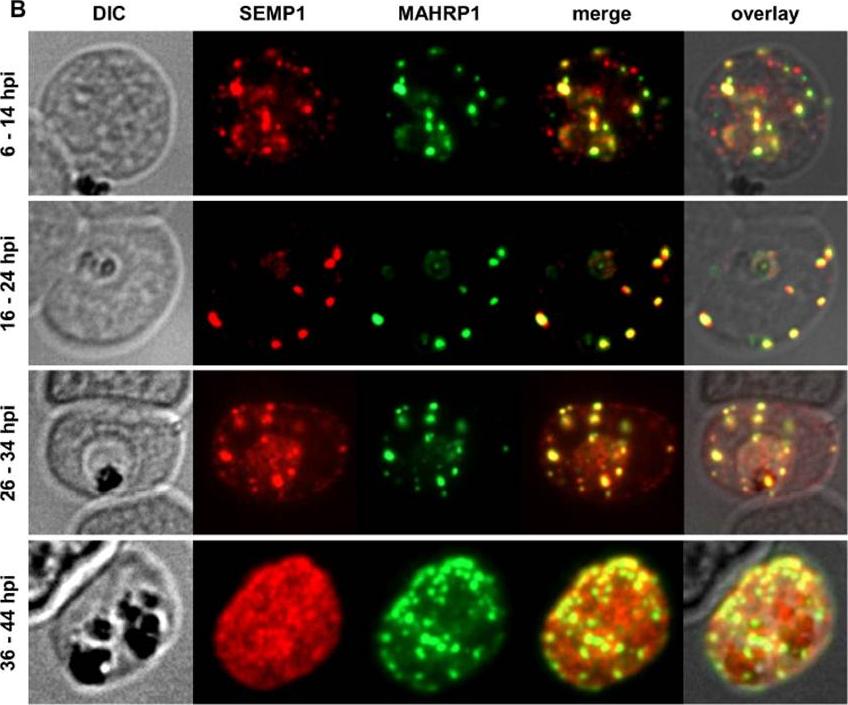
Localization and expression of endogenous SEMP1 in 3D7 wild-type parasites. IFAs of MeOH-fixed RBCs infected with 3D7 wild-type parasites synchronized at timepoints 6–14 hours post invasion (hpi), 16–24 hpi, 26–34 hpi and36–44 hpi. Cells were co-labelled with mouse a-SEMP1 and rabbit a-MAHRP1 serum. Dual-labelling IFAs of sorbitol-synchronized parasites at 6–14 hours post invasion (hpi), 16–24 hpi, 26–34 hpi, and 36–44 hpi showed that SEMP1, similarly to MAHRP1, is exported early after invasion to MCs where it partially co-localized with MAHRP1Dietz O, Rusch S, Brand F, Mundwiler-Pachlatko E, Gaida A, Voss T, Beck HP. Characterization of the Small Exported Plasmodium falciparum Membrane Protein SEMP1. PLoS One. 2014 9(7):e103272.
See original on MMP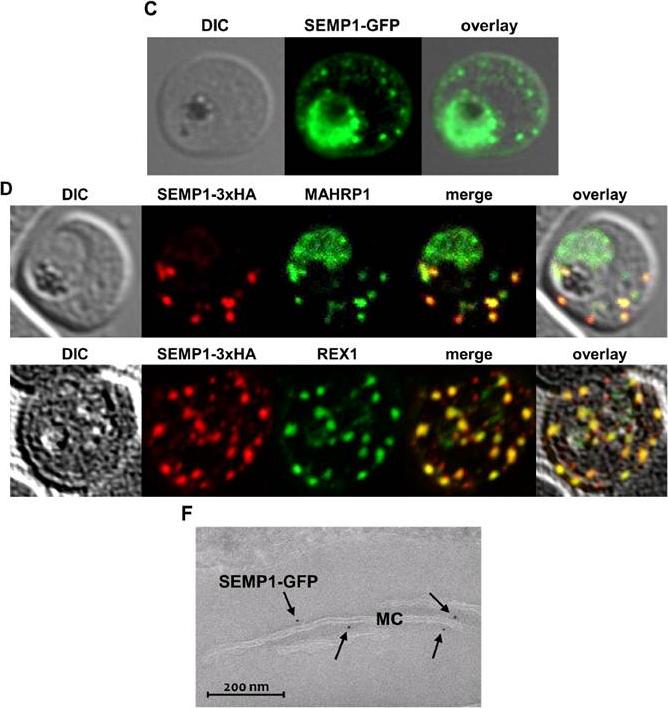
C: Live cell imaging of 3D7 parasites expressing SEMP1 with C-terminal tagged GFP. D: Immunofluorescence assays of MeOHfixed RBCs infected with 3D7 expressing SEMP1 with a C-terminal 3xHA tag, co-labelled with rat a-HA and either rabbit a-MAHRP1 or rabbit a-REX1 antibodies. D: Scatter plot of co-localization of SEMP1 and REX1 in SEMP1-3xHA parasites. E: Electron microscopy (EM) of RBCs infected with 3D7 expressing SEMP1 with a C-terminal GFP tag labelled with rabbit a-GFP antibodies and decorated with 5 nm gold conjugated Protein A. GFP-tagged protein was exported (c). SEMP1-3xHA co-localized with MAHRP1 and REX1 to the MCs. Electron microscopy using antibodies against GFP confirmed the localization of SEMP1-GFP at the MCs (F). Dietz O, Rusch S, Brand F, Mundwiler-Pachlatko E, Gaida A, Voss T, Beck HP. Characterization of the Small Exported Plasmodium falciparum Membrane Protein SEMP1. PLoS One. 2014 9(7):e103272.
See original on MMP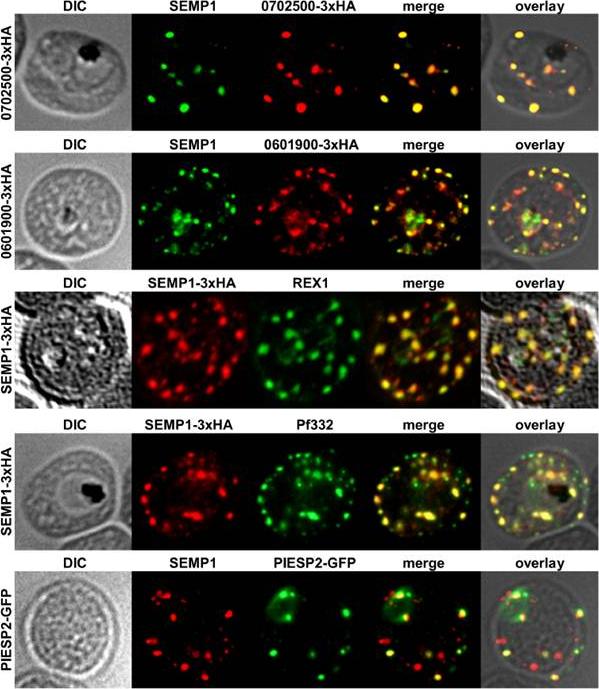
Localization of potential SEMP1 interacting proteins. Immunofluorescence assays of MeOH-fixed RBCs infected with 3D7 expressing EMP1/PF3D7_0702500 (PF07_0008)/PF3D7_0601900 (PFF0090w) with a C-terminal 3xHA tag and 3D7 expressing PIESP2 with a C-terminal GFP-tag, co-labelled with mouse a-SEMP1 and rat a-HA (PF3D7_0702500-3xHA & PF3D7_0601900-3xHA) /a-GFP (PIESP2-GFP). For co-labelling of SEMP1-3xHA with REX1 and Pf332, rat a-HA and rabbit a-REX1 / mouse a-Pf332 antibodies were used. All potential interaction partners co-localized at least partially with SEMP1, but in particular with Pf332 or PIESP2 antibodies only a fraction of SEMP1 positive MCs were labelled with either antibody suggesting that distinct subpopulations of MCs exist. Dietz O, Rusch S, Brand F, Mundwiler-Pachlatko E, Gaida A, Voss T, Beck HP. Characterization of the Small Exported Plasmodium falciparum Membrane Protein SEMP1. PLoS One. 2014 9(7):e103272.
See original on MMP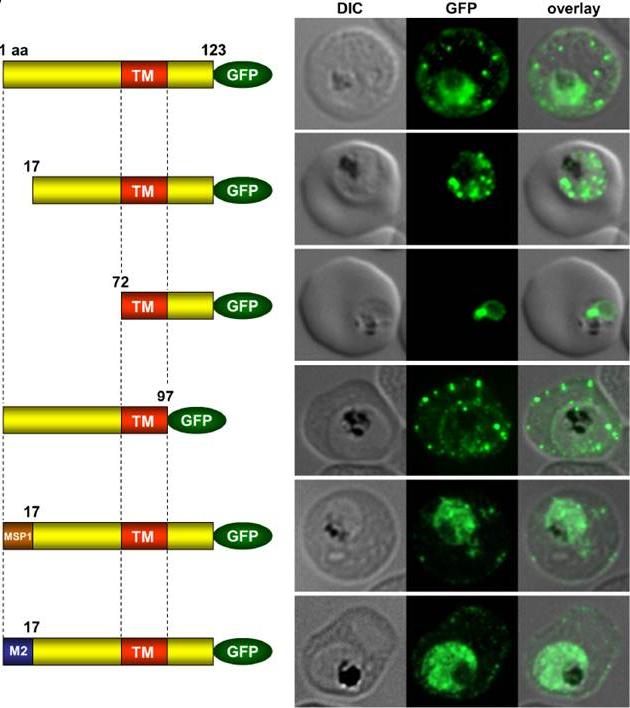
Immunofluorescence assays of MeOH-fixed RBCs infected with SEMP1-KO parasites expressing full-length and truncated or mutated forms of SEMP1 C-terminally fused to GFP. Expressed SEMP1 was labelled with rabbit a-GFP antibodies. The transmembrane domain is depicted in red (TM), a MSP1 signal peptide in brown (MSP1) and the MAHRP2 N-terminus in blue (M2). deletion of the complete C-terminus of SEMP1 (SEMP11–97-GFP) did not impair export and resulted in correct localisation whilst deletion of the first 16 amino acids (SEMP117–123-GFP) of the N-terminus resulted in an impaired export from the parasite. The deletion of 71 amino acids (SEMP172–123-GFP) of the N-terminus prevented export and seemed to have resulted in concentration of SEMP1 in or at the endoplasmic reticulum. If the first 16 amino acids of SEMP1 were replaced by the corresponding amino acids of MAHRP2 (M21–16SEMP117–123-GFP) or the classical signal peptide of MSP1 (MSP11–16SEMP117–123-GFP) the fusion protein was exported in both cases albeit with lower efficiency.Dietz O, Rusch S, Brand F, Mundwiler-Pachlatko E, Gaida A, Voss T, Beck HP. Characterization of the Small Exported Plasmodium falciparum Membrane Protein SEMP1. PLoS One. 2014 9(7):e103272.
See original on MMPMore information
| PlasmoDB | PF3D7_0702400 |
| GeneDB | PF3D7_0702400 |
| Malaria Metabolic Pathways | Localisation images Pathways mapped to |
| Previous ID(s) | PF07_0007 |
| Orthologs | |
| Google Scholar | Search for all mentions of this gene |