PF3D7_0201900 erythrocyte membrane protein 3 (EMP3)
Disruptability [+]
| Species | Disruptability | Reference | Submitter |
|---|---|---|---|
| P. falciparum 3D7 |
Possible |
USF piggyBac screen (Insert. mut.) | USF PiggyBac Screen |
| P. falciparum 3D7 |
Possible |
10856227 Truncation |
Theo Sanderson, Francis Crick Institute |
Mutant phenotypes [+]
| Species | Stage | Phenotype | Reference | Submitter |
|---|---|---|---|---|
| P. falciparum 3D7 | Asexual |
Altered cytoadherence |
10856227 Truncation |
Theo Sanderson, Francis Crick Institute |
Imaging data (from Malaria Metabolic Pathways)
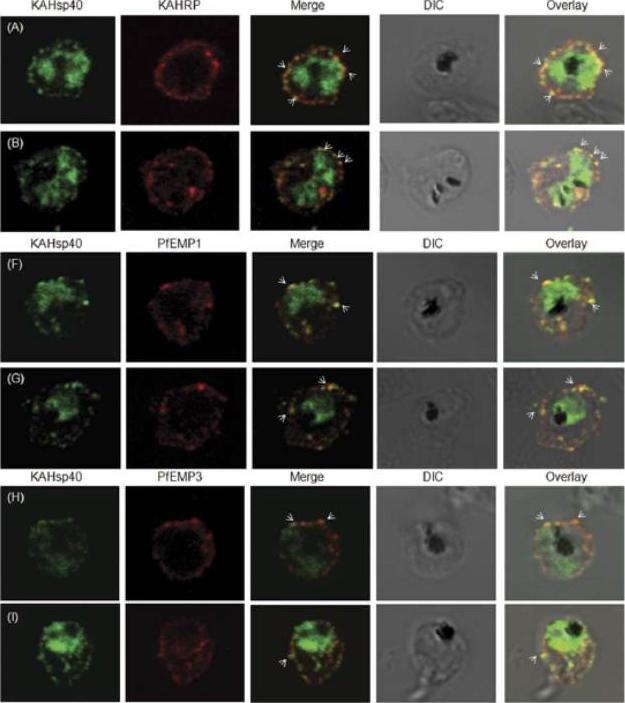
KAHsp40 associates with knobs on the infected erythrocyte membrane. (A-B) IFA reveals that KAHsp40 and KAHRP co-localize in the erythrocyte periphery near the erythrocyte plasma membrane. White arrows indicate the discrete foci in which they co-localize. KAHsp40 and PfEMP1 and KAHsp40 and PfEMP3 co-localize with each other on the erythrocyte plasma membrane indicating the close association of KAHsp40 with knobs.Acharya P, Chaubey S, Grover M, Tatu U. An Exported Heat Shock Protein 40 Associates with Pathogenesis-Related Knobs in Plasmodium falciparum Infected Erythrocytes. PLoS One. 2012;7(9):e44605.
See original on MMP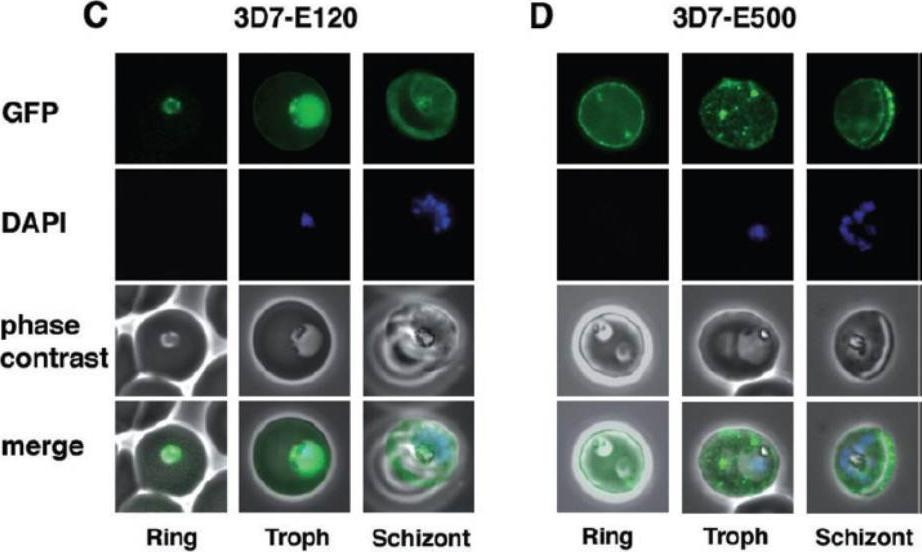
Detection of live GFP fluorescence in the PfEMP3-GFP P. falciparum transgenic lines. 3D7-E120 (C) and 3D7-E500 (D). Shown for each transgenic line is a representative image of a ring stage-infected RBC, a trophozoite stage-infected RBC and a schizont stage-infected RBC. The first row in each panel depicts the native GFP fluorescence, the second row the nucleus stained with DAPI, the third row the phase contrast image and the forth row the overlays of all three images. parasites expressing E120-GFP also generally showed an even distribution although there was some apparent concentration at the periphery of the infected RBC (C). In contrast, 3D7-E500 parasites showed very strong peripheral concentration of the GFP chimera under the RBC membrane and associated with punctuate structures within the RBC cytosol (D).Knuepfer E, Rug M, Klonis N, Tilley L, Cowman AF. Trafficking determinants for PfEMP3 export and assembly under the Plasmodium falciparum-infected red blood cell membrane. Mol Microbiol. 2005 58:1039-53. Erratum in: Mol Microbiol. 2006 59:722. PubMed PMID:
See original on MMP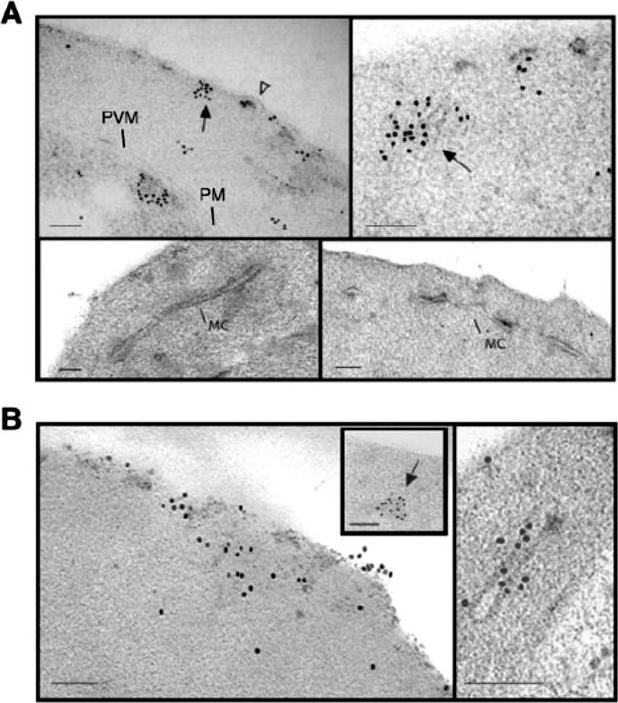
Subcellular localization of E120-GFP and E500-GFP in transgenic 3D7 parasite lines. Immuno-electron microscopy studies of 3D7-E120 the N-terminal 120 amino acids attached to GFP (A) and 3D7-E500 the N-terminal 500 amino acids attached to GFP. (B) P. falciparum transgenic parasites labelled with rabbit αGFP antibody. In both cases gold particles were detected in association with the RBC membrane and electron-dense material within the RBC cytoplasm (closed arrows). In 3D7-E120 transfectants the GFP chimera seems to associate underneath knob structures (open arrow) similar to wild-type PfEMP3. However, whereas E500-GFP can be seen associated with Maurer’s clefts, E120-GFP does not associate with these structures. Bars represent 100 nm.Knuepfer E, Rug M, Klonis N, Tilley L, Cowman AF. Trafficking determinants for PfEMP3 export and assembly under the Plasmodium falciparum-infected red blood cell membrane. Mol Microbiol. 2005 58:1039-53. Erratum in: Mol Microbiol. 2006 59:722.
See original on MMP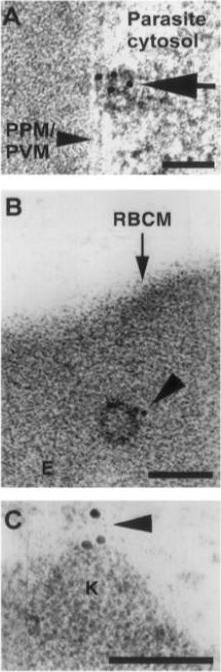
Trophozoite stage IRBC were processed for immunogold labeling and probed with a rat anti-PfEMP-3 IgG followed by a goat anti-rat IgG coupled to 10 nm gold. PfEMP-3 was distributed on electron-dense vesicles which appear to fuse with the parasite plasma membrane (A, large arrow), on the cytoplasmic leaflet of electron-dense vesicles within the erythrocyte cytosol (E) (B, arrowhead), as well as on the knob structures (K) on the erythrocyte plasma membrane (RBCM) (C, arrowhead). K, knob; RBCM, erythrocyte plasma membrane; E, erythrocyte cytosol; PPM, parasite plasma membrane; PVM, parasite vacuolar membrane. Scale bar100 nm.Trelka DP, Schneider TG, Reeder JC, Taraschi TF. Evidence for vesicle-mediated trafficking of parasite proteins to the host cell cytosol and erythrocyte surface membrane in Plasmodium falciparum infected erythrocytes. Mol Biochem Parasitol. 2000 106:131-45. Copyright Elsevier 2009.
See original on MMP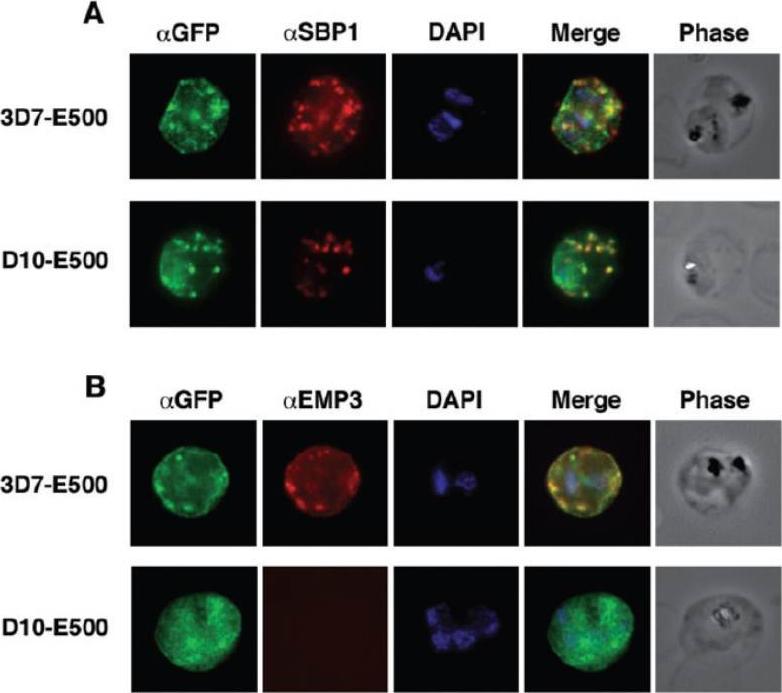
Colocalization of E500-GFP chimeric protein with a Maurer’s clefts marker and native PfEMP3. Indirect mmunofluorescence assays were performed on trophozoite stages of E120-GFP-expressing transgenic 3D7 and E500-GFP-expressing transgenic 3D7 and D10 P. falciparum lines. A. Formaldehyde fixed trophozoite stages were labelled with rabbit αGFP (first column; green), mouse α PfSBP1, a Maurer’s clefts localized protein (second column; red) and DAPI for nuclear staining (third column; blue). The forth column represents the overlay of all three images and the fifth column the phase contrast image of the infected RBC. B. E500-GFP-expressing parasites were fixed and labelled with rabbit αGFP (first column; green), mouse αPfEMP3 (second column; red), an antibody raised against a part of the 3′ repeat region V and DAPI for nuclear staining (third column; blue). The forth column shows the overlays of the three images and the fifth column the phase contrast image of the infected erythrocyte. E120-GFP does not associate with Maurer’s clefts. E500-GFP and PfSBP1, however, colocalize in Maurer’s cleft structures in 3D7-E500. Knuepfer E, Rug M, Klonis N, Tilley L, Cowman AF. Trafficking determinants for PfEMP3 export and assembly under the Plasmodium falciparum-infected red blood cell membrane. Mol Microbiol. 2005 58:1039-53. Erratum in: Mol Microbiol. 2006 59:722
See original on MMP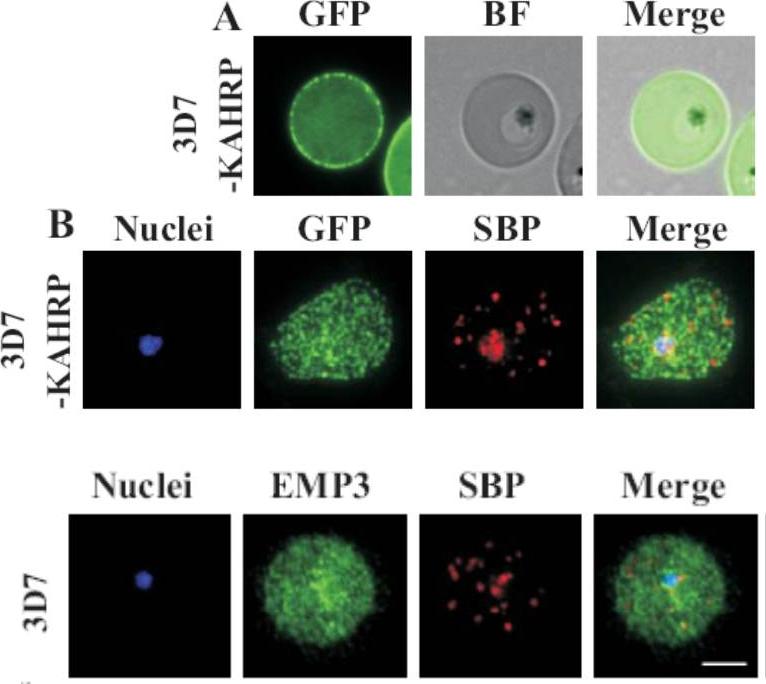
(A) live cell fluorescence microscopy of 3D7-KAHRP-GFP. Panels are GFP fluorescence, bright field (BF) and a merge of the two. Scale bar: 5 μm. KAHRP-GFP was trafficked to the RBC membrane and gave a rim fluorescence pattern (B) Immunofluorescence microscopy of acetone-fixed RBCs infected with 3D7-KAHRP-GFP, SBP1 is a marker for the Maurer’s clefts. Lower row: Immunofluorescence microscopy of acetone fixed RBCs infected with wild type. Smears were probed with mouse anti-PfEMP3 (green) and rabbit anti-SBP1 (red). A characteristic PfEMP3 profile at the RBC membrane is seen.Dixon MW, Kenny S, McMillan PJ, Hanssen E, Trenholme KR, Gardiner DL, Tilley L. Genetic ablation of a Maurer's cleft protein prevents assembly of the Plasmodium falciparum virulence complex. Mol Microbiol. 2011 81(4):982-93.
See original on MMP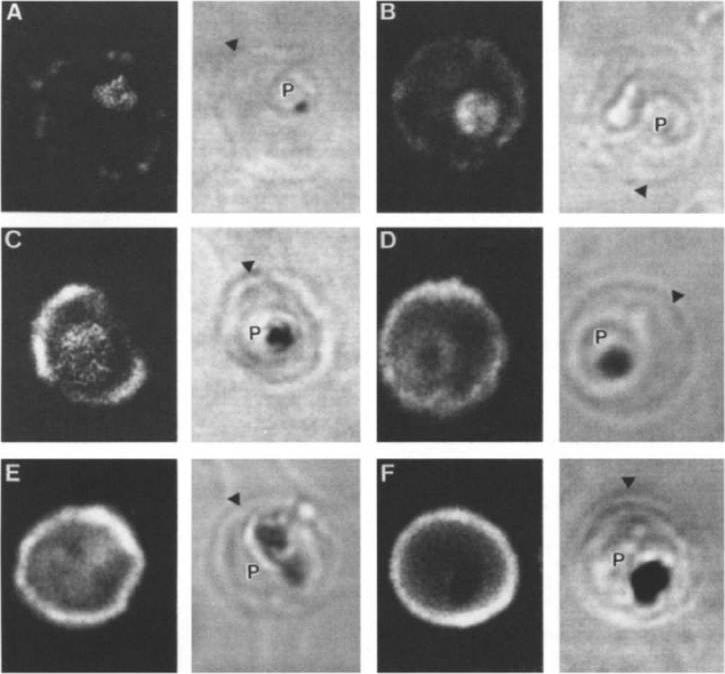
Stage analysis of HRPl (KAHRP) and PfEMP3 synthesis in PE by IFA and confocal microscopy. Both the MC K+ and the FCR-3 K+ parasites were analyzed, each producing the same fluorescent patterns. HRPl was detected with mAb89 and PfEMP3 was detected using chicken anti-PfEMP3 polyclonal antibody. The images in the first (fluorescent) and second (transmitted light) columns are using anti-HRPl antibody. The images in the third (fluorescent) and fourth (transmitted light) columns are using anti-PfEMP3 antibody. Panels A and B, ring stage (12-15 h); panels C and D, early- to mid-trophozoite stage (24-30 h); panels E and F, schizont stage (40-48 h). The arrowheads designate the erythrocyte membrane of the parasitized eryhtrocyte. Pasloske BL, Baruch DI, Ma C, Taraschi TF, Gormley JA, Howard RJ. PfEMP3 and HRP1: co-expressed genes localized to chromosome 2 of Plasmodium falciparum. Gene. 1994 144:131-6. Copyright Elsevier 2010.
See original on MMP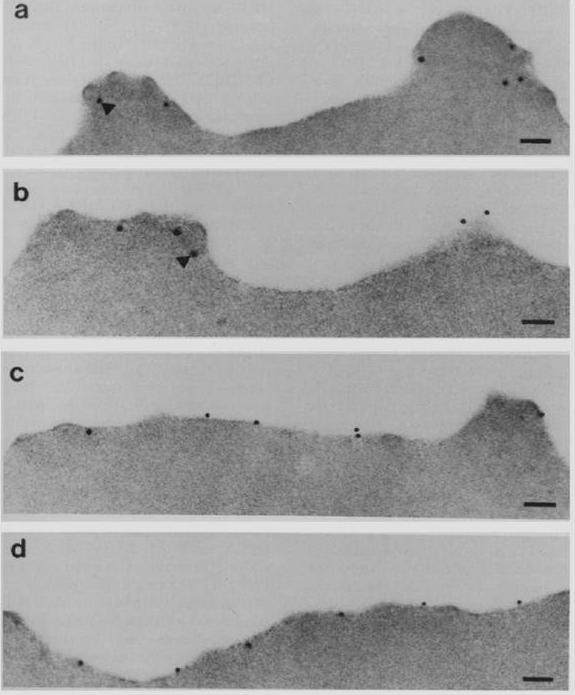
Subcellular localization of PfEMP3 and HRP1 (KAHRP) by immunoelectron microscopy. Fixed trophozoite PE embedded in LR white resin were incubated with either mAb 89 KAHRP (A and B) or mouse anti-RP serum PfEMP3 (C and D). In panels A and B, the arrows indicate the gold particles considered below the erythrocyte membrane. Bars, 0.1 mm.Pasloske BL, Baruch DI, van Schravendijk MR, Handunnetti SM, Aikawa M, Fujioka H, Taraschi TF, Gormley JA, Howard RJ. Cloning and characterization of a Plasmodium falciparum gene encoding a novel high-molecular weight host membrane-associated protein, PfEMP3. Mol Biochem Parasitol. 1993 59:59-72. Copyright Elsevier 2009.
See original on MMP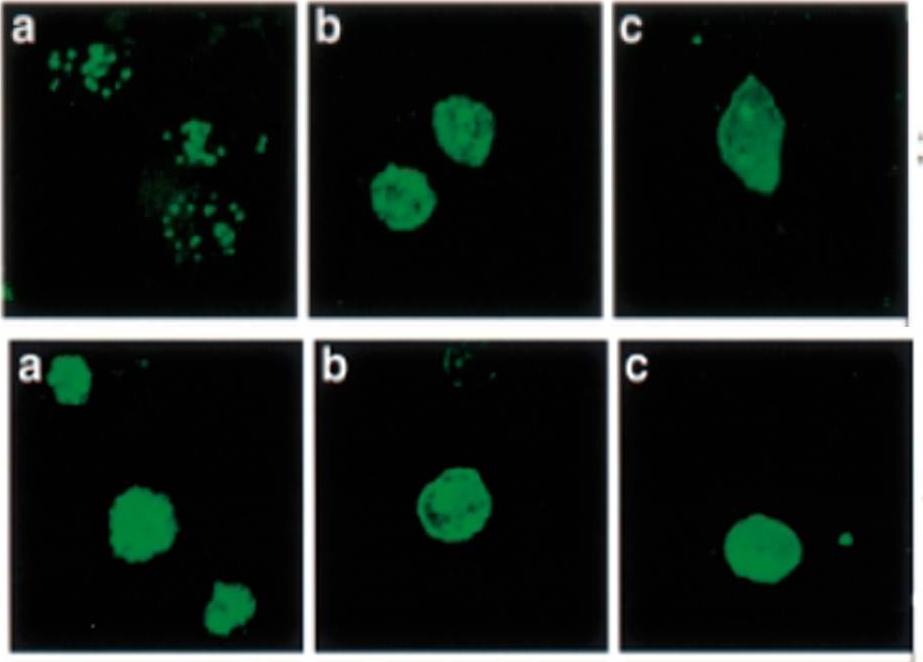
Immunolocalization of PfEMP1 (a), PfEMP3 (b) and KAHRP (c) in rings (upper row) and mature torphozoites (lower row) using specific antibodies and visualization by confocal microscopy. PfEMP3 is deposited under the membrane of the infected RBC. Mutation of PfEMP3 disrupts transfer of PfEMP1 to the outside of infected cells.Adapted by permission from Macmillan Publishers Ltd: Waterkeyn JG, Wickham ME, Davern KM, Cooke BM, Coppel RL, Reeder JC, Culvenor JG, Waller RF, Cowman AF. Targeted mutagenesis of Plasmodium falciparum erythrocyte membrane protein 3 (PfEMP3) disrupts cytoadherence of malaria-infected red blood cells. EMBO J. 2000 19:2813-23.
See original on MMP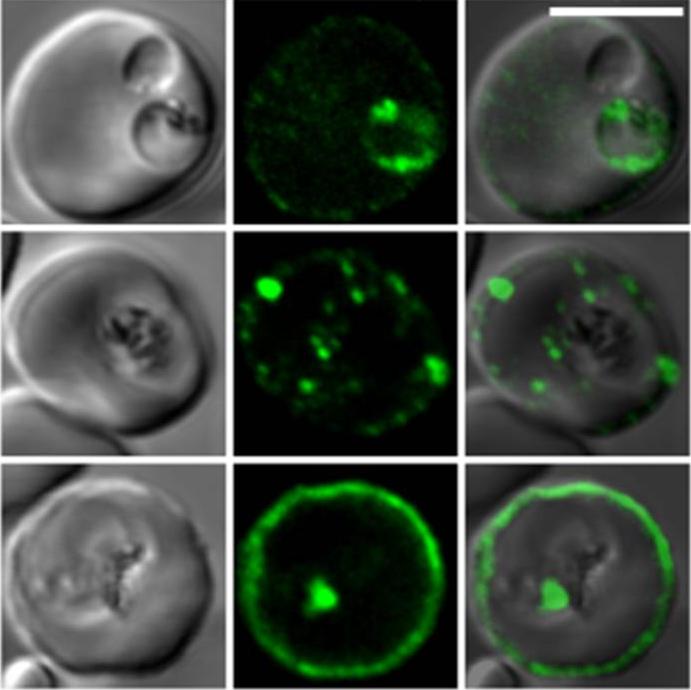
Confocal fluorescence microscopy images of transfected 3D7 P. falciparum-infected RBCs expressing a GFP chimera directed to the red blood cell membrane skeleton. DIC image, the GFP fluorescence signal and an overlay of a P. falciparum erythrocyte membrane protein-31-500 -GFP transfectant. Scale bar = 5 µm.PfEMP3 is present in the parasite's endomembrane system in the ring stage (top row), and with the Maurer’s clefts (middle row) and red blood cell membrane skeleton (bottom row) in more mature stage parasites.Tilley L, McFadden G, Cowman A, Klonis N. Illuminating Plasmodium falciparum-infected red blood cells. Trends Parasitol. 2007 23:268-77. Copyright Elsevier 2009.
See original on MMP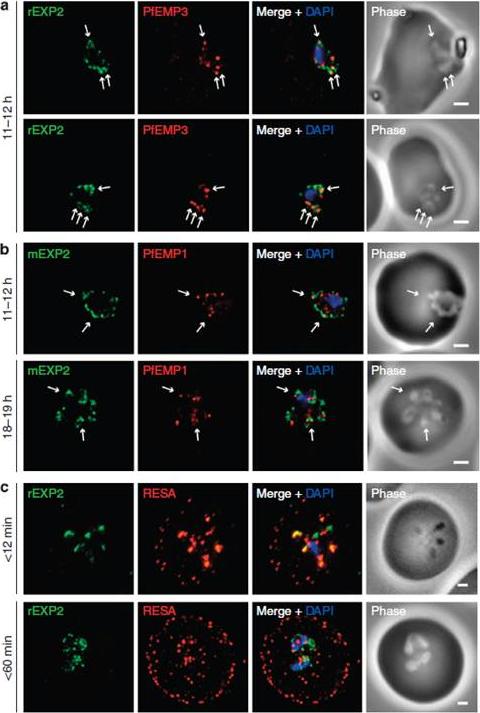
Protein accumulates at sites marked by PTEX during native PEXEL-dependent protein export. Widefield deconvolution microscopy of parasites fixed and labelled by IFA for EXP2 (green), the nucleus (40,6–diamidino-2-phenylindole (DAPI), blue) and (a) PfEMP3 (red), a protein exported by PEXEL-dependent processes, or (b) PfEMP1, a protein likely to undergo PEXEL-independent export, showed some areas of possible association of both exported proteins and EXP2; however, results were inconclusive. Parasite ages are as shown. Scale bars, 1 mm. Parasites were labelled by IFA for EXP2 (green), RESA (red) and the nucleus (DAPI, blue) having been (c) fixed o12 min or 60–90 min after invasion and imaged by widefield deconvolution microscopy (scale bar, 1 mm).Riglar DT, Rogers KL, Hanssen E, Turnbull L, Bullen HE, Charnaud SC, Przyborski J, Gilson PR, Whitchurch CB, Crabb BS, Baum J, Cowman AF. Spatial association with PTEX complexes defines regions for effector export into Plasmodium falciparum-infected erythrocytes. Nat Commun. 2013 Jan 29;4:1415.
See original on MMP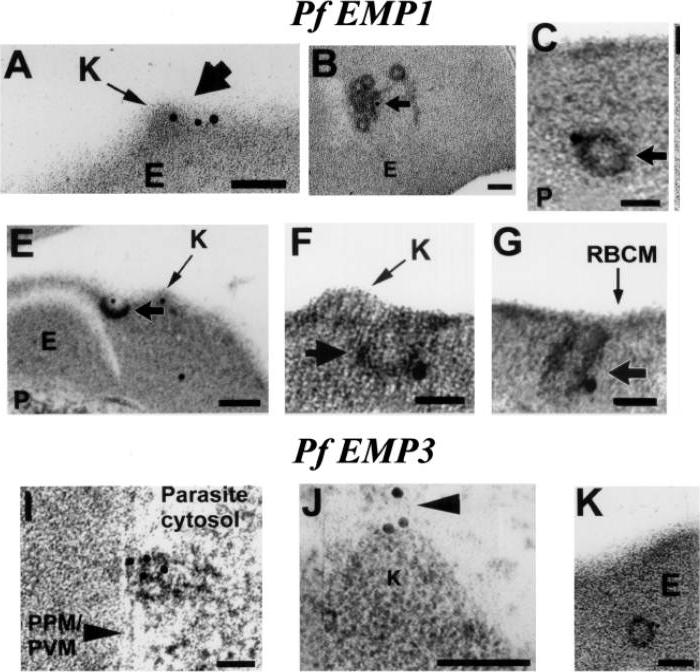
Evidence that the electron dense vesicles within the erythrocyte cytosol transport PfEMP1 and PFEMP3 to the erythrocyte membrane. Trophozoite stage IRBC were processed for immunoelectron microscopy and probed with a rabbit a-PfEMP1 IgG followed by a goat a-rabbit IgG coupled to 15 nm gold. PfEMP1 is distributed on knobs (K) (A, short arrow), on electron dense vesicles that appear to fuse with the red blood cell membrane (E–G, short arrow) and among coated, electron dense vesicles in the erythrocyte cytosol (B,C, short arrow). Trophozoite stage IRBC were processed for immunoelectron microscopy and probed with a rat PfEMP3 IgG followed by a goat a-rat IgG coupled to 10 nm gold. PfEMP3 is distributed on electron dense vesicles that appear to fuse with the parasite plasma membrane (I, arrowhead), on the cytoplasmic leaflet of electron dense vesicles within the erythrocyte cytosol (K) and on knobs (J, arrowhead). Scale bar = 100 nm.Taraschi TF, Trelka D, Martinez S, Schneider T, O'Donnell ME. Vesicle-mediated trafficking of parasite proteins to the host cell cytosol and erythrocyte surface membrane in Plasmodium falciparum infected erythrocytes. Int J Parasitol. 2001 31:1381-1391. Copyright Elsevier 2010.
See original on MMP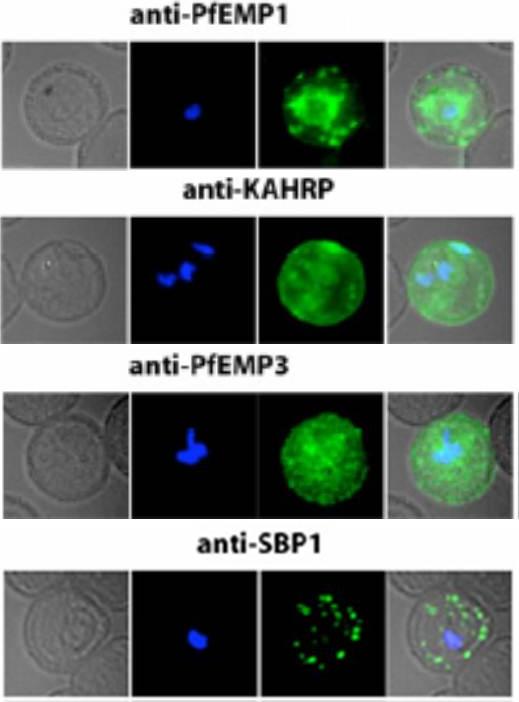
Localization of PfEMP1, KAHRP, PfEMP3 and SBP1 in P. falciparum-infected erythrocytes. The first column of each panel shows a bright-field image, the second panel specific antibody and the third panel overlay of the previous two and a nuclear stain (DAPI). PfEMP1 displays and functions on the surface of infected erythrocytes. KAHRP and PfEMP3 are exported to the erythrocyte cytoplasm and interacts with Maurer’s clefts and eventually reach the inner side of the host cell membrane. SBP1 is a Maurer’s clefts resident protein.Maier AG, Rug M, O'Neill MT, Brown M, Chakravorty S, Szestak T, Chesson J, Wu Y, Hughes K, Coppel RL, Newbold C, Beeson JG, Craig A, Crabb BS, Cowman AF. Exported proteins required for virulence and rigidity of Plasmodium falciparum-infected human erythrocytes. Cell. 2008 134:48-61.
See original on MMP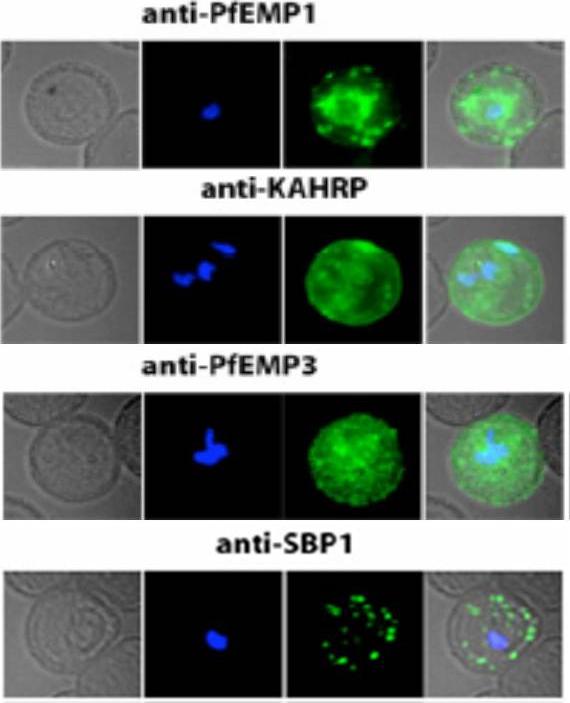
Localization of PfEMP1, KAHRP, PfEMP3 and SBP1 in P. falciparum-infected erythrocytes. The first column of each panel shows a bright-field image, the second panel specific antibody and the third panel overlay of the previous two and a nuclear stain (DAPI). PfEMP1 displays and functions on the surface of infected erythrocytes. KAHRP and PfEMP3 are exported to the erythrocyte cytoplasm and interacts with Maurer’s clefts and eventually reach the inner side of the host cell membrane. SBP1 is a Maurer’s clefts resident protein.Maier AG, Rug M, O'Neill MT, Brown M, Chakravorty S, Szestak T, Chesson J, Wu Y, Hughes K, Coppel RL, Newbold C, Beeson JG, Craig A, Crabb BS, Cowman AF. Exported proteins required for virulence and rigidity of Plasmodium falciparum-infected human erythrocytes. Cell. 2008 134:48-61.
See original on MMP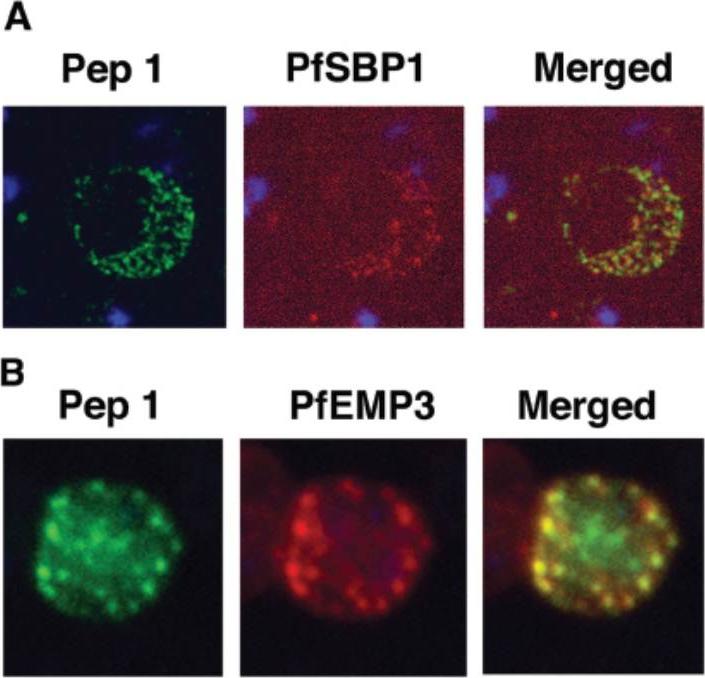
Colocalization studies with anti-peptide 1 antibodies and fixed 3D7 pRBC from schizont ghosts and PfEMP3 truncated 3D7 cell lines. (A) Schizont ghosts. (B) PfEMP3 truncated 3D7 cell lines. Parasite nuclei were stained with DAPI (blue). Double staining was carried out with anti-peptide 1 rabbit serum (green) and mouse serum (B28) against the 77-kDa carboxy terminus of MC protein PfSBP1 (red) or mouse serum (DG662) against the carboxy terminus of PfEMP3 (red). Anti-peptide 1 serum was detected by using FITC-conjugated goat anti-rabbit IgG. Colocalizing antibody was detected by using Texas red-conjugated goat anti-mouse IgG. Magnification, x1,000. A truncated form of PfEMP-3 obtained by transfection was no longer distributed around the cytoplasmic surface of the erythrocyte membrane but was instead associated with structures believed to be MC. IFA experiments with antibodies against PfEMP-3 and STEVOR showed the colocalization of truncated PfEMP-3 and STEVOR (B), further supporting the observation that STEVOR is located in MC.Kaviratne M, Khan SM, Jarra W, Preiser PR. Small variant STEVOR antigen is uniquely located within Maurer's clefts in Plasmodium falciparum-infected red blood cells. Eukaryot Cell. 2002 1:926-35.
See original on MMP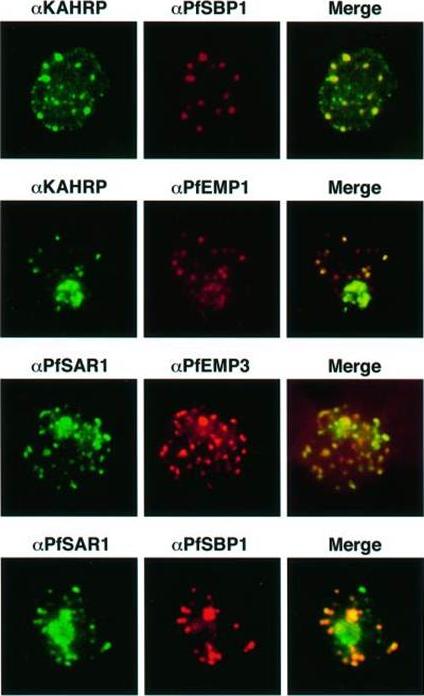
Transient co-localization of KAHRP, PfSBP1, PfEMP1, PfEMP3 and PfSAR1p to Maurer’s clefts in ring stage P.falciparum-infected erythrocytes. First row: co-localization of KAHRP (green) with PfSBP1 (red). Second row: co-localization of KAHRP (green) with PfEMP1 (red). Third row: co-localization of PfSAR1p (green) with PfEMP3 (red). Fourth row: co-localization of PfSAR1p (green) with PfSBP1 (red). The third window in each row represents the merging of the red and green channels. Yellow areas represent regions of co-localization.Wickham ME, Rug M, Ralph SA, Klonis N, McFadden GI, Tilley L, Cowman AF. Trafficking and assembly of the cytoadherence complex in Plasmodium falciparum-infected human erythrocytes. EMBO J. 2001 20(20):5636-49.
See original on MMP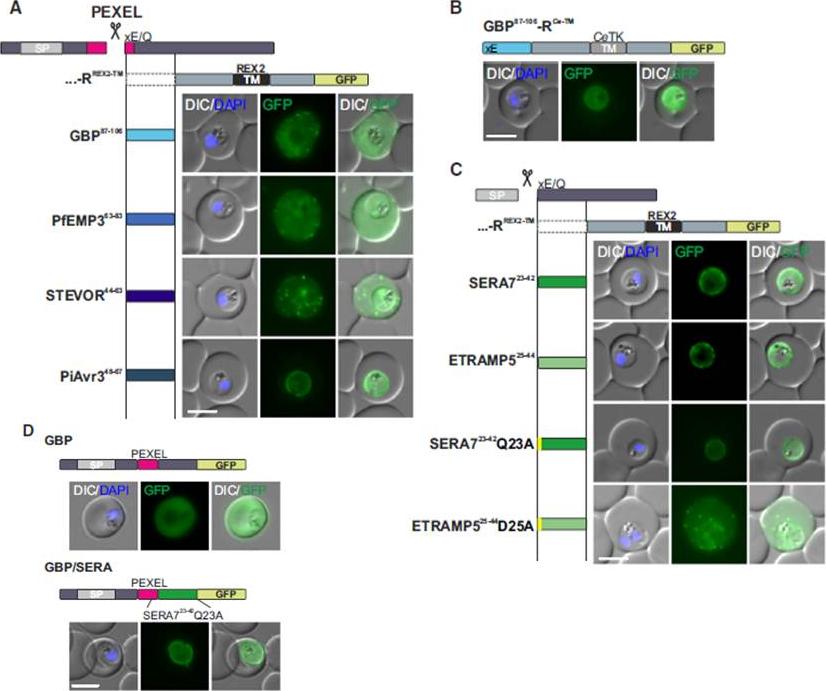
Mature PEXEL N Termini Promote Export of RREX2-TM (A–C) Images of live P. falciparum parasites expressing RREX2-TM fused with the mature N termini of PEXEL proteins (A), GBP87–106-RCe-TM (B), or RREX2-TM fused with the mature N termini of nonexported secretory proteins (C). The position of the appended region in the original protein is shown above each panel.(D) Images of live P. falciparum parasites expressing truncated GBP fused to GFP (GBP, top) or GBP-GFP with the mature N terminus of SERA7Q23A after the PEXEL (GBP/SERA, bottom). Although these N termini contain only the last of the conserved PEXEL residues (PEXEL position 5) and the nonconserved position 4, they promoted export of the reporter into the host cell (A). GFP fluorescence was detected in the erythrocyte cytosol and the Maurer’s clefts.Grüring C, Heiber A, Kruse F, Flemming S, Franci G, Colombo SF, Fasana E,Schoeler H, Borgese N, Stunnenberg HG, Przyborski JM, Gilberger TW, Spielmann T. Uncovering common principles in protein export of malaria parasites. Cell Host Microbe. 2012 12(5):717-29.
See original on MMP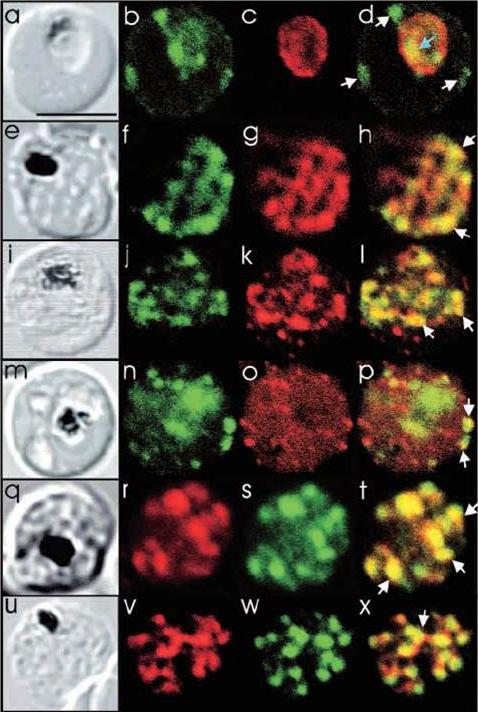
Comparison of the location of PfSec31p with that of other parasite antigens in P. falciparum-infected erythrocytes. (a-d) Asynchronous infected erythrocytes (K1 strain) were probed with affinity-purified rabbit anti-PfSec31(WD) antiserum followed by a fluorescein-conjugated anti-rabbit IgG (b; green fluorescence) and a murine monoclonal antibody recognising the PV-located protein Exp1 followed by rhodamine conjugated anti-mouse IgG (c; red fluorescence). An optical slice was collected through the centre of the parasite by confocal microscopy. Some PfSec31p-containing structures in the erythrocyte cytosol are marked with white arrowheads and a PfSec31p-containing structure in the parasite cytosol is marked with a blue arrowhead (d). (a) A transmission image of (b-d). (e-l) Asynchronous infected erythrocytes (K1 strain) were probed with mouse anti-PfSec31 (WD) antiserum followed by a fluorescein-conjugated anti-mouse IgG (green fluorescence) and a rabbit anti-PfSar1p antiserum followed by Alexa-Fluor-568-conjugated anti-rabbit IgG (red fluorescence). Optical slices were collected near the surface of the parasitised erythrocytes. Some PfSec31p- and PfSar1p-containing structures are marked with arrowheads. (m-p) Asynchronous infected erythrocytes were probed with affinity-purified rabbit anti-PfSec31( WD) antiserum followed by fluorescein-conjugated anti-rabbit IgG (green fluorescence) and a murine monoclonal antibody recognising PfEMP3, followed by Alexa-Fluor-568-conjugated anti-mouse IgG (red florescence). An optical slice was collected near the surface of the parasitised erythrocyte. Two structures in which there is partial overlap of PfSec31p and PfEMP3 in the erythrocyte cytosol are marked with arrowheads. (q-x) Asynchronous infected erythrocytes were probed with a mouse anti-PfSec31(WD) antiserum followed by Alexa-Fluor-568-conjugated anti-mouse IgG (red fluorescence) and an affinity-purified rabbit anti-PfEMP1 antibody followed by fluorescein-conjugated anti-rabbit IgG (green florescence). Optical slices were collected near the surface of the parasitised erythrocytes. Some PfSec31p- and PfEMP1-containing structures in the erythrocyte cytosol are marked with arrowheads. Bar in (a), 5 mm.Adisa A, Albano FR, Reeder J, Foley M, Tilley L. Evidence for a role for a Plasmodium falciparum homologue of Sec31p in the export of proteins to the surface of malaria parasite-infected erythrocytes. J Cell Sci. 2001 114(Pt 18):3377-86.
See original on MMP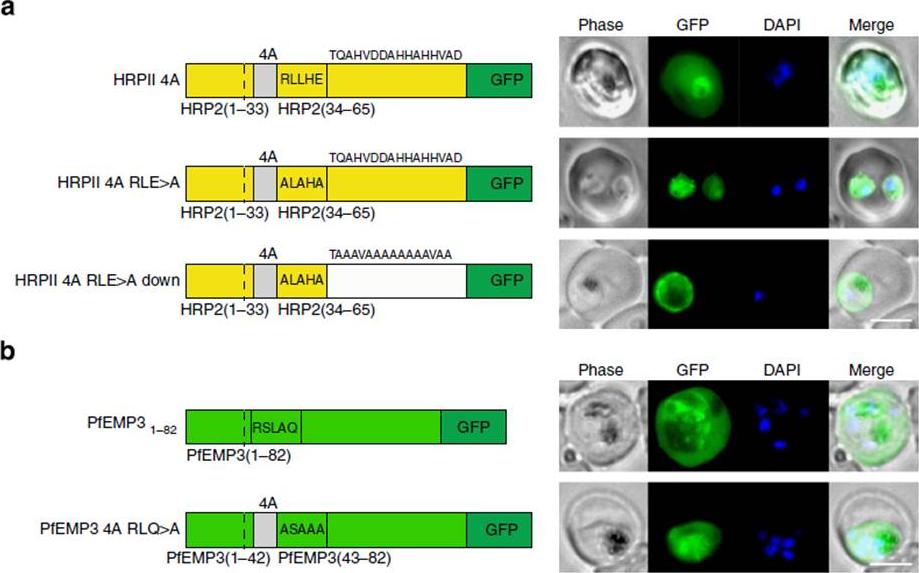
PEXEL-independent export of PEXEL proteins does not occur in P. falciparum. (a) HRPII 4A is exported to the P. falciparum-infected erythrocyte. HRPII 4A RLE4A and HRPII 4A RLE4A Down are not exported. Scale bar, 5 mm. (b) PfEMP31–82 is exported by P. falciparum to the erythrocyte, but PfEMP3 4A RLQ4A is not. Scale bar, 5 mm.Boddey JA, O'Neill MT, Lopaticki S, Carvalho TG, Hodder AN, Nebl T, Wawra S, van West P, Ebrahimzadeh Z, Richard D, Flemming S, Spielmann T, Przyborski J, Babon JJ, Cowman AF. Export of malaria proteins requires co-translational processing of the PEXEL motif independent of phosphatidylinositol-3-phosphate binding. Nat Commun. 2016 Feb 1;7:10470.
See original on MMP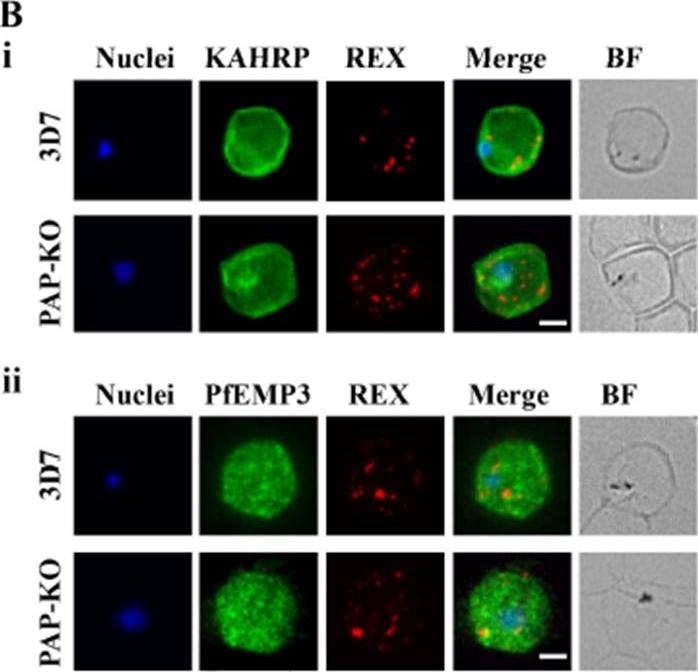
Targeted gene disruption of PfPAP changes the filterability of the infected RBCs but not the expression or localization of KAHRP, REX1, and PfEMP-3. B). Immunofluorescence using anti-KAHRP, REX1, and PfEMP-3 antibodies on 3D7 and 3D7_PAP KO show no difference in the location of these proteins. C). Electron microscopy of 3D7_PAP-KO showing electron dense structures at the surface of the infected erythrocyte indicative of the presence of knobs. Bars are 5 mm.da Silva FL, Dixon MW, Stack CM, Teuscher F, Taran E, Jones MK, Lovas E, Tilley L, Brown CL, Trenholme KR, Dalton JP, Gardiner DL, Skinner-Adams TS. A Plasmodium falciparum S33 proline aminopeptidase is associated with changes in erythrocyte deformability. Exp Parasitol. 2016 Jun 30. pii:S0014-4894(16)30135-7.
See original on MMPMore information
| PlasmoDB | PF3D7_0201900 |
| GeneDB | PF3D7_0201900 |
| Malaria Metabolic Pathways | Localisation images Pathways mapped to |
| Previous ID(s) | PF02_0019, PFB0095c |
| Orthologs | |
| Google Scholar | Search for all mentions of this gene |