PCHAS_1412300 V-type proton ATPase catalytic subunit A, putative
Disruptability [+]
| Species | Disruptability | Reference | Submitter | |
|---|---|---|---|---|
| P. berghei ANKA |
Refractory |
PlasmoGEM (Barseq) | PlasmoGEM | |
| P. falciparum 3D7 |
Possible |
USF piggyBac screen (Insert. mut.) | USF PiggyBac Screen | |
Mutant phenotypes [+]
None reported yet. Please press the '+' button above to add one.Imaging data (from Malaria Metabolic Pathways)
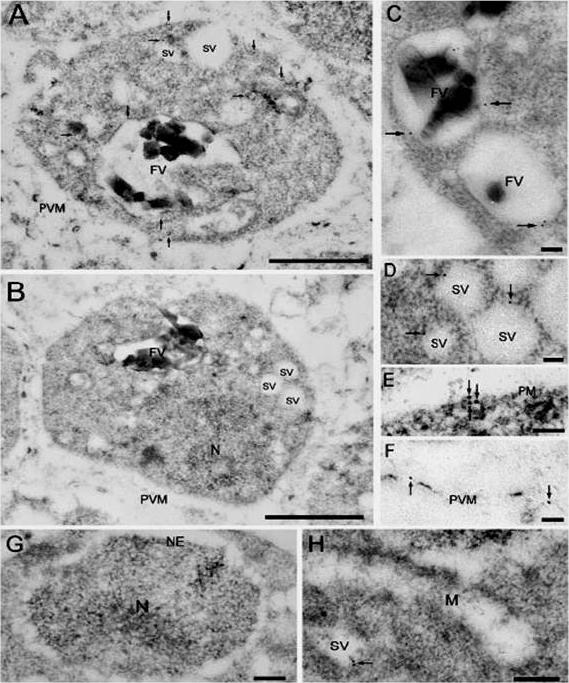
Localization of V-ATPase subunit A. A and B show the whole body of the parasite immunostained with anti-V-ATPase subunit A antibodies (A) or control IgG (B); C, parts of food vacuoles (FV); D, small clear vesicles (SV); E, plasma membrane (PM); F, parasitophorus vacuolar membrane (PVM); G, nucleus (N) and nuclear envelope (NE); H, mitochondrion (M); Immunogold for V-ATPase subunit A selectively labeled food vacuoles (A and C), small clear vesicles (A and D), and the plasma membrane (A and E). Immunogold particles were also occasionally observed in the parasitophorus vacuolar membrane (F),Hayashi M, Yamada H, Mitamura T, Horii T, Yamamoto A, Moriyama Y. Vacuolar H+-ATPase localized in plasma membranes of malaria parasite cells, Plasmodium falciparum, is involved in regional acidification of parasitized erythrocytes. J Biol Chem. 2000 275:34353-8.
See original on MMP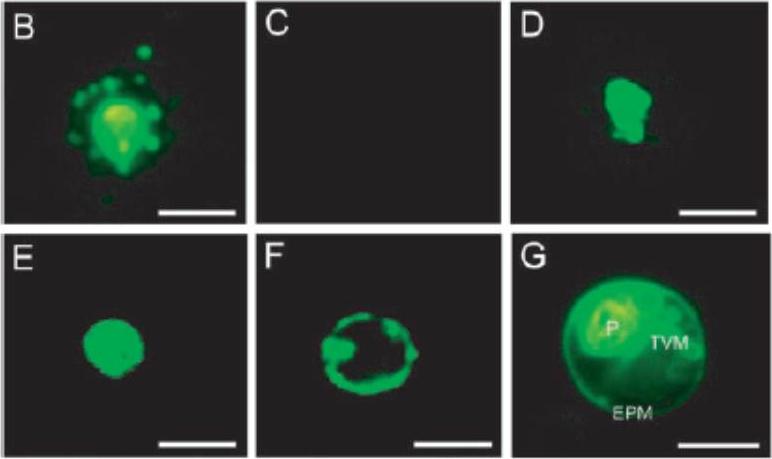
Immunohistochemical detection of PfNSF. (B), PfNSF in noninfected erythrocyte (C), vacuolar H1-ATPase subunit PF13_0065 A (V-ATPase) (D), H1-pumping pyrophosphatase (V-PPase) PF14_0541 (E), and the serine repeat antigen protein (F) in parasitized erythrocytes. The parasitized erythrocytes were immunostained with the indicated antibodies and then observed by fluorescence microscopy. Vital staining with C5-ceramide was also performed to reveal localization of the tubovesicular membrane networks (TVM) (G). P, P. falciparum cell; EMP, erythrocyte plasma membrane. Bar, 5 mm. the PfNSF immunoreactivity was present within the vesicular structures outside the parasite cells (B). No such extraparasitized vesicular structures were observed in the immunoreactivities against antibodies for vacuolar H+-ATPase (D), H+-pumping pyrophosphatase (E), serine repeat antigen protein, markers for the peripheral space between the parasitophorus vacuolar membranes, and the plasma membrane of the malaria parasite (F).Hayashi M, Taniguchi S, Ishizuka Y, Kim HS, Wataya Y, Yamamoto A, Moriyama Y. A homologue of N-ethylmaleimide-sensitive factor in the malaria parasite Plasmodium falciparum is exported and localized in vesicular structures in the cytoplasm of infected erythrocytes in the brefeldin A-sensitive pathway. J Biol Chem. 2001 May 4;276(18):15249-55.
See original on MMPMore information
| PlasmoDB | PCHAS_1412300 |
| GeneDB | PCHAS_1412300 |
| Malaria Metabolic Pathways | Localisation images Pathways mapped to |
| Previous ID(s) | PC001143.02.0, PC001409.02.0, PCAS_141230, PCHAS_141230 |
| Orthologs | PBANKA_1410400 , PF3D7_1311900 , PKNH_1412600 , PVP01_1412900 , PVX_122430 , PY17X_1412200 |
| Google Scholar | Search for all mentions of this gene |