PCHAS_0419100 ras-related protein RAB7, putative (RAB7)
Disruptability [+]
| Species | Disruptability | Reference | Submitter | |
|---|---|---|---|---|
| P. berghei ANKA |
Refractory |
PlasmoGEM (Barseq) | PlasmoGEM | |
| P. falciparum 3D7 |
Refractory |
USF piggyBac screen (Insert. mut.) | USF PiggyBac Screen | |
Mutant phenotypes [+]
None reported yet. Please press the '+' button above to add one.Imaging data (from Malaria Metabolic Pathways)
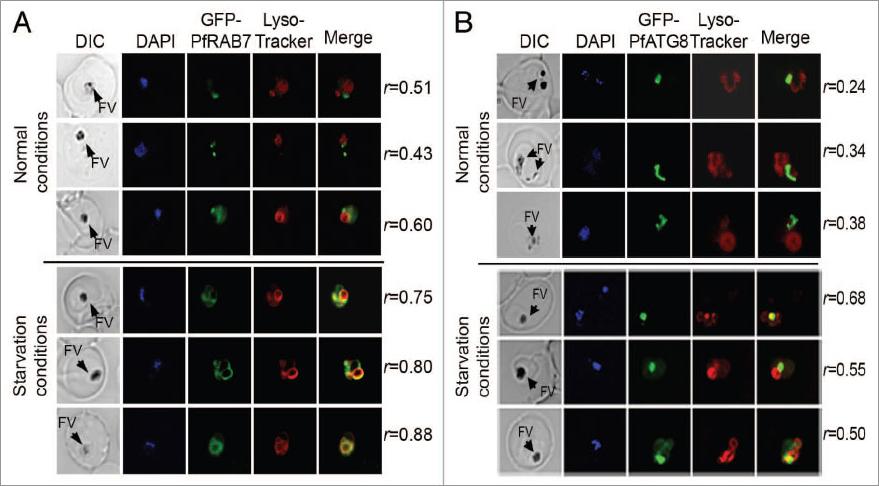
In vivo association between GFP-PfRAB7 and GFP-PfATG8 and acidic compartments. Distribution of GFP-PfRAB7 (A) or GFP-PfATG8 (B) and acidic compartments revealed in red with LysoTracker Red-DND99 is observed under normal and starvation conditions using live cell microscopy [Leica DMI 6000 microscope with MicroMAX-1300Y/HS camera (Princeton Instruments)]. The association of GFP-PfRAB7 and acidic compartments is shown in the merged image (yellow). Comparisons are between parasites at similar stages of development. The Pearson’s coefficient (r) represents the degree of colocalization in the individual images. FV; Food vacuoleTomlins AM, Ben-Rached F, Williams RA, Proto WR, Coppens I, Ruch U, Gilberger TW, Coombs GH, Mottram JC, Müller S, Langsley G. Plasmodium falciparum ATG8 implicated in both autophagy and apicoplast formation. Autophagy. 2013 Aug 29;9(10).
See original on MMP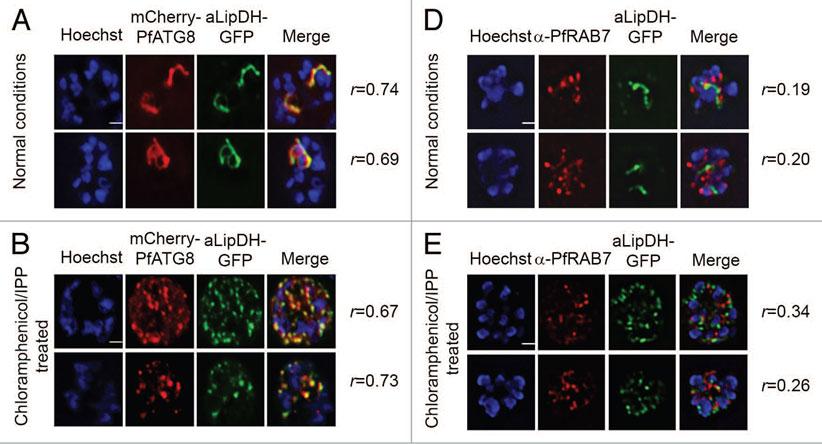
PfATG8, but not PfRAB7, colocalizes with apicoplast-targeted vesicles and apicoplast remnants. (A and B) P. falciparum expressing both mCherry-PfATG8 and aLipDH-GFP (lipoamide dehydrogenase) were treated with chloramphenicol to chemically disrupt the formation of the apicoplast, and IPP added to the culture medium to maintain viability of the parasites. DMSO control (A) and chloramphenicol treated (B) infected erythrocytes were counterstained with Hoechst 33258. The Pearson’s coefficients (r) show the degree of colocalization in each individual image. (D and E) Transgenic parasites expressing aLipDH-GFP were treated as above. Immunofluorescence analyses of untreated controls (D) or chloramphenicol/IPP treated (E) and were performed using anti-PfRAB7 antibodies (red). Hoechst 33258 (blue) was used to stain the nuclei. Scale bar: 2 μm.Tomlins AM, Ben-Rached F, Williams RA, Proto WR, Coppens I, Ruch U, Gilberger TW, Coombs GH, Mottram JC, Müller S, Langsley G. Plasmodium falciparum ATG8 implicated in both autophagy and apicoplast formation. Autophagy. 2013 Aug 29;9(10). PMID: 2402567
See original on MMP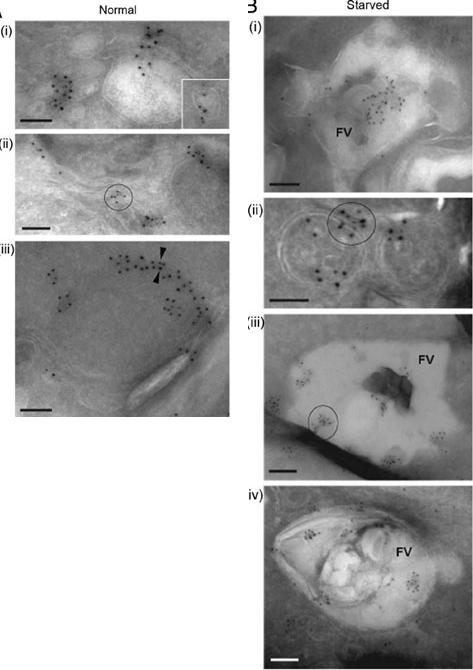
Left-normal: A iii) and PfRAB7 (5-nm gold particles, A iii). (A) PfATG8 and PfRAB7 decorate vesicles (i and insert) and membranous tubules (ii) in the cytoplasm. On some occasions, Immunogold staining of P. falciparum under normal growth conditions and under conditions of amino acid-deprivation (starved) reveals the subcellular localization of PfATG8 (10-nm gold particlesPfATG8 and PfRAB7 occurred at the periphery of double-membrane-bound organelles (iii, arrowheads). Scale bars: 100 nm. PfATG8 and PfRAB7-labeled vesicles (i and insert) and membranous tubules (ii) and PfATG8 also occurred at the periphery of double membrane bound organelles (iii, arrowheads).Right-starved: PfATG8 and PfRAB7 associatedwith large structures corresponding to the food vacuole (FV; i). Costaining of PfATG8 and PfRAB7 is also visible on small double-membrane-boundstructures (ii), or very large FV vesicles (iii and iv). Scale bars: 100 nm. Upon amino acid deprivation an enhanced colocalization of PfATG8 and PfRAB7with the acidic food vacuole (FV) occurred (i, iii and iv) and also on small double-membrane-bound structures (ii).Tomlins AM, Ben-Rached F, Williams RA, Proto WR, Coppens I, Ruch U, Gilberger TW, Coombs GH, Mottram JC, Müller S, Langsley G. Plasmodium falciparum ATG8 implicated in both autophagy and apicoplast formation. Autophagy. 2013 Aug29;9(10).
See original on MMP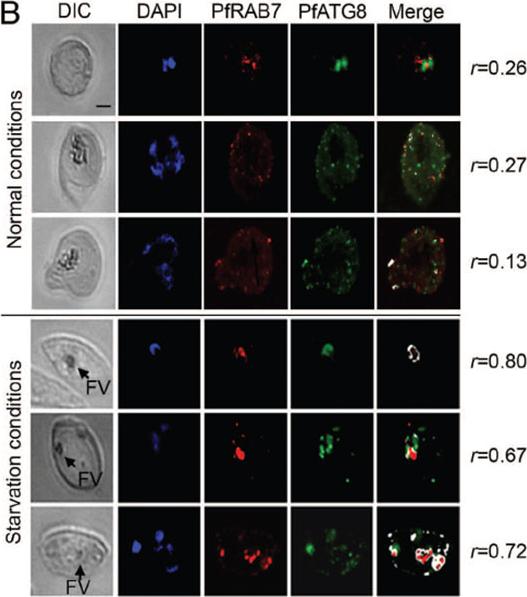
Amino acid deprivation induces fusion between PfRAB7-positive and PfATG8-positive compartments. Association of PfRAB7 and PfATG8 (PF10_0193) under normal or amino acid-deprivation conditions using IFA. The distribution of PfRAB7 (red: excitation: 530 to 560 nm and emission 572 to 647 nm; anti-PfRAB7 peptide antibody) and PfATG8 (green: excitation: 450 to 490 nm and emission 500 to 550 nm; anti-PfATG8 peptide antibody) is shown. Nuclei are stained with DAPI (blue: excitation: 340 to 380 nm and emission 450 to 490 nm). The association of PfRAB7 and PfATG8 is shown in white (merge). The images were obtained using a Leica DMI 6000 microscope with MicroMAX-1300Y/HS camera (Princeton Instruments). The Pearson’s coefficient (r) represents the degree of colocalization between PfRAB7 and PfATG8 in the individual images. FV: food vacuole. In nonstarved parasites, PfATG8, but not PfRAB7, was found on the intact apicoplast membrane and on apicoplast-targeted vesicles. Amino acid starvation provoked increased colocalization between PfATG8- and PfRAB7-labeled vesicles and acidification of the colabeled structures consistent with PfRAB7-mediated maturation of PfATG8-positive autophagosomes.Tomlins AM, Ben-Rached F, Williams RA, Proto WR, Coppens I, Ruch U, Gilberger TW, Coombs GH, Mottram JC, Müller S, Langsley G. Plasmodium falciparum ATG8 implicated in both autophagy and apicoplast formation. Autophagy. 2013 Aug29;9(10). [Epub ahead of print]
See original on MMP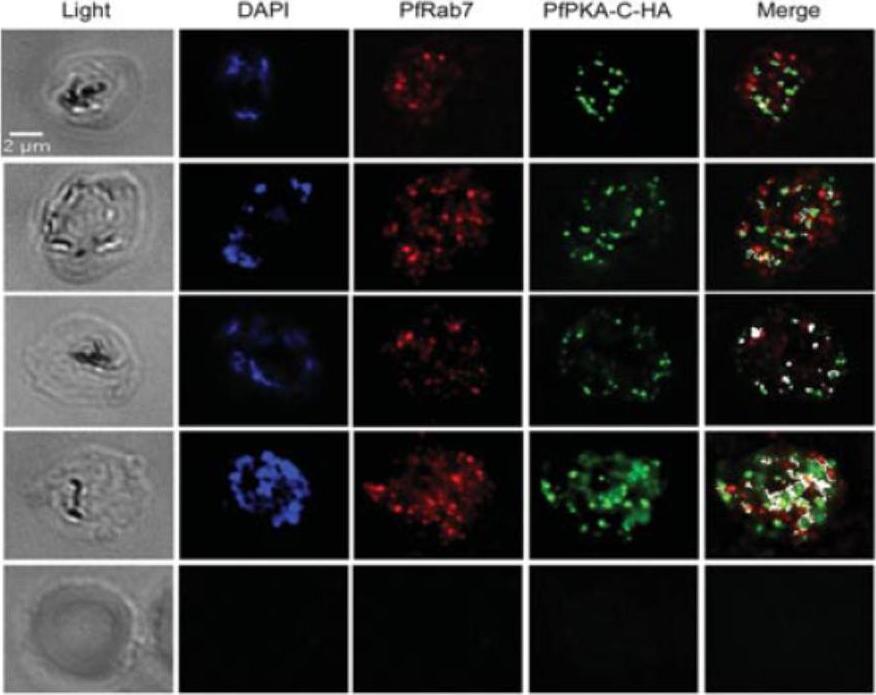
Association between PfRab7 and PfPKA-C-HA in vivo is shown at a single stack of image acquisition. The distribution of PfRab7 (red) is revealed with a specific anti-peptide PfRab7 antibody and the distribution of PfPKA-C-HA (green) is revealed with anti-HA antibodies. The association between PfRab7 and PfPKA-C-HA is shown in white. The level of association between Rab7 and PfPKA-C-HA increases as the parasite develops into a multi-nucleated schizont (nuclei shown stained in blue with DAPI). One can observe a subset of PfRab7+ vesicles associated with a subset of PfPKA-C-HA+ structures. The ability of PfRab7 to bind PfPKA-C suggests that it acts somewhat like a PKA-anchor proteins (AKAP), recruiting PKA to early and late endosomes.Rached FB, Ndjembo-Ezougou C, Chandran S, Talabani H, Yera H, Dandavate V, Bourdoncle P, Meissner M, Tatu U, Langsley G. Construction of a Plasmodium falciparum Rab-interactome identifies CK1 and PKA as Rab-effector kinases in malaria parasites. Biol Cell. 2012 104(1):34-47.
See original on MMP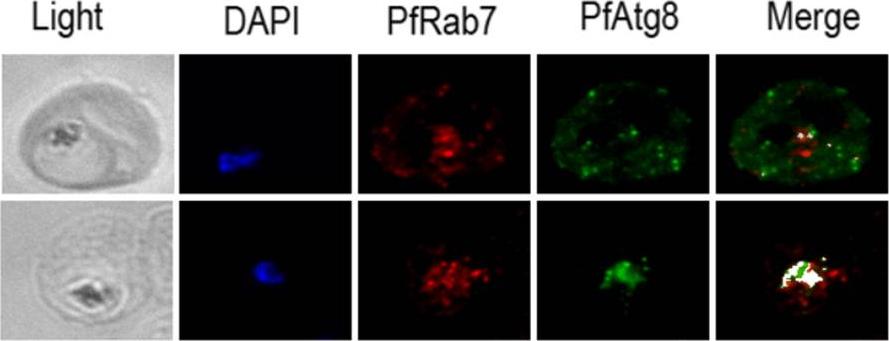
Immunofluorescence showing colocalization (white) of PfRab7 (red) and PfAtg8 (green) in normal and starvation culture conditions. PfRab7+/PfAtg8+ double labeled autophagosomes can be found at the food vacuole suggesting that it, rather than lysosomes, is the final lytic compartment in malaria parasite autophagy.Ben-Rached F & Langsley G (2014) Rab GTPases in Plasmodium. These figures were published in the Encyclopedia of Malaria edited by Marcel Hommel and Peter G. Kremsner (Springer New York)
See original on MMP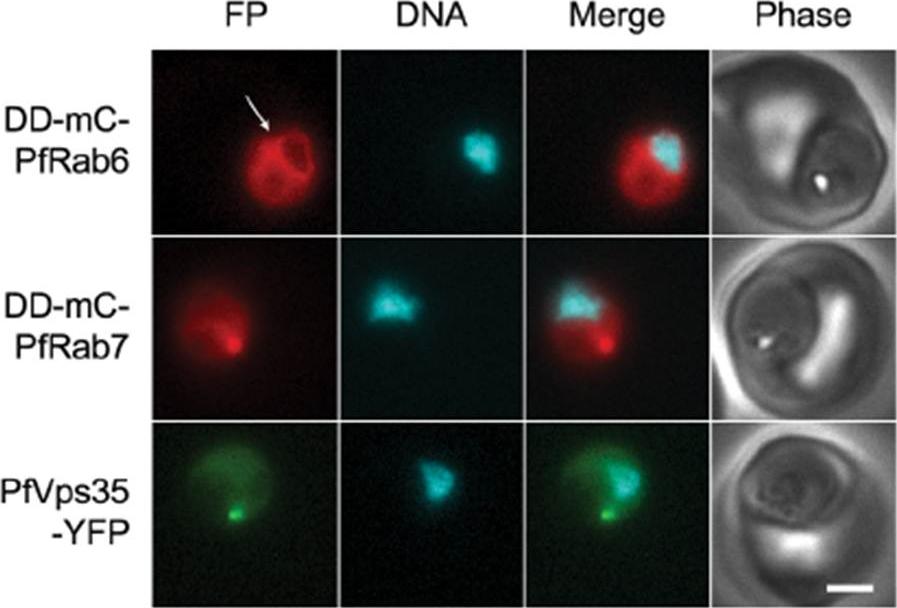
PfRab6 but not PfRab7 or retromer rapidly redistributes to the ER upon brefeldin A treatment. (A) Distributions of DDmCherry-PfRab6, DD-mCherry-PfRab7 and PfVps35-YFP in live parasites after one hour in the presence of 5 mg/mL brefeldin A. Redistribution of DDmCherry-PfRab6 to the perinuclear ER is indicated with an arrow (top panel). FP, fluorescent protein. mCherry fluorescence is pseudocolored red, YFP fluorescence is pseudocolored green and Hoechst 33342 fluorescence is pseudocolored cyan. Scale bar, 2 mm. Golgi marker DD-mCherry-PfRab6 redistributed to the perinuclear ER. Association with the perinuclear ER was not observed for either Rab7 or Vps35. The contrasting responses of the Golgi apparatus and endosome to brefeldin A treatment reveal differences in the dynamics of vesicular traffic to and from these compartments.Krai P, Dalal S, Klemba M. Evidence for a Golgi-to-Endosome Protein Sorting Pathway in Plasmodium falciparum. PLoS One. 2014 9(2):e89771.
See original on MMP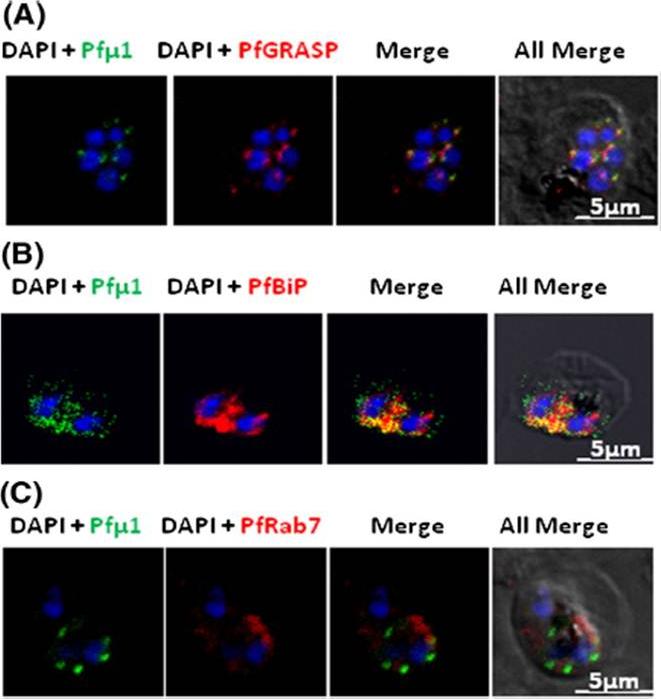
Pfμ1 is closely associated with the Golgi and ER in early trophozoite stages. Transgenic parasites expressing Pfμ1–GFP protein at trophozoite stages were immunostainedwith anti-PfGRASP (A), anti-PfBiP (B) and anti-PfRab7 (C) antibodies. The parasite nuclei were stained with DAPI and slides were visualized by confocal laser scanning microscopy. Scale bars denote 5 μm. Pfμ1 partially co-localized with PfGRASP and PfBip. the ER (which is contiguous with the nuclear envelope) and Golgi in developing merozoites are very closely juxtaposed, and dense vesicular traffic occurs between these two compartments, as well as between nascent rhoptries. No overlap between Pfμ1–GFP and Rab7.This suggests that these proteins do not co-localize, and hence that Rab7 is not involved in trafficking at this stage (3C). These results thus suggest the presence of Pfμ1 in the Golgi–ER network during the early stages of intra-erythrocytic development.Kaderi Kibria KM, Rawat K, Klinger CM, Datta G, Panchal M, Singh S, Iyer GR, Kaur I, Sharma V, Dacks JB, Mohmmed A, Malhotra P. A role for adaptor protein complex 1 in protein targeting to rhoptry organelles in Plasmodium falciparum. Biochim Biophys Acta. 2015 1853(3):699-710.
See original on MMPMore information
| PlasmoDB | PCHAS_0419100 |
| GeneDB | PCHAS_0419100 |
| Malaria Metabolic Pathways | Localisation images Pathways mapped to |
| Previous ID(s) | PC000223.00.0, PCAS_041910, PCHAS_041910 |
| Orthologs | PBANKA_0418200 , PF3D7_0903200 , PKNH_0700900 , PVP01_0701600 , PVX_098605 , PY17X_0421000 |
| Google Scholar | Search for all mentions of this gene |