PBANKA_1409100 ras-related protein Rab-5B, putative (RAB5b)
Disruptability [+]
| Species | Disruptability | Reference | Submitter | |
|---|---|---|---|---|
| P. berghei ANKA |
Refractory |
RMgm-822 | Imported from RMgmDB | |
| P. berghei ANKA |
Refractory |
PlasmoGEM (Barseq) | PlasmoGEM | |
| P. falciparum 3D7 |
Possible |
USF piggyBac screen (Insert. mut.) | USF PiggyBac Screen | |
Mutant phenotypes [+]
None reported yet. Please press the '+' button above to add one.Imaging data (from Malaria Metabolic Pathways)
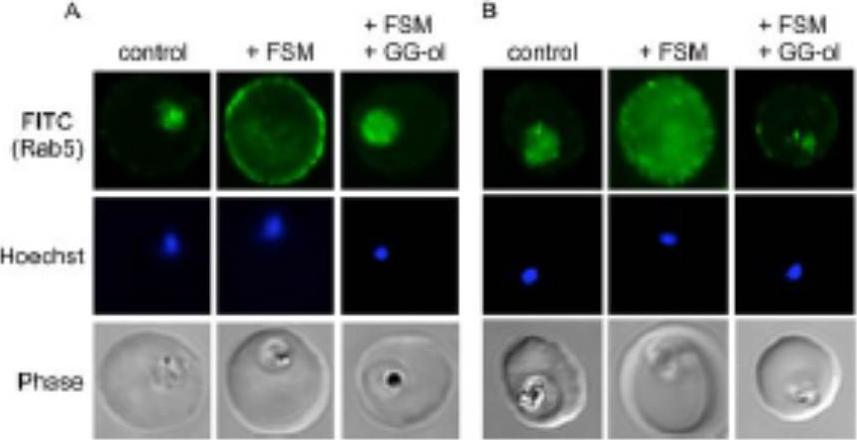
Confocal immunofluorescence with either anti-PfRab5a (A) or anti-PfRab5c (B) in untreated parasites (control) compared to fosmidomycin (+ FSM) and fosmidomycin + geranylgeraniol (+FSM +GG-ol) treated parasites. Pf-Rab5a and Pf-Rab5c were dispersed in punctae throughout malaria parasite cells. Upon fosmidomycin treatment, a dramatic mislocalization of Rab5a occurred in the majority of treated cells, such that Rab5a was no longer present within the parasite cell, but instead was found at the membrane of the host erythrocyte.Howe R, Kelly M, Jimah J, Hodge D, Odom AR. Isoprenoid biosynthesis inhibition disrupts Rab5 localization and food vacuolar integrity in Plasmodium falciparum. Eukaryot Cell. 2013 12(2):215-23 .
See original on MMP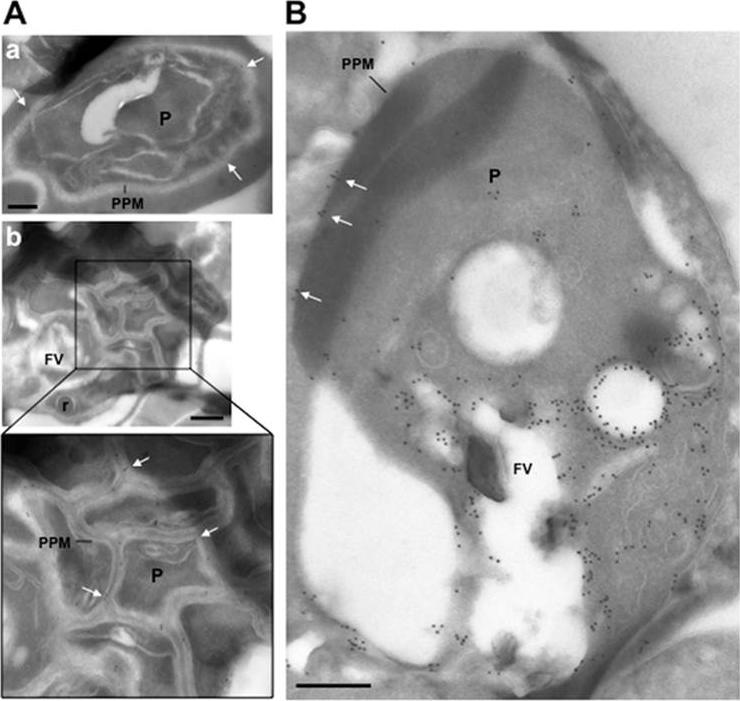
Ultrastructural detection of PfRab5b in P. falciparum-infected RBC. Immuno-electron microscopy of schizonts (A) and a large trophozoite (B) of P. falciparum labelled with specific anti-PfRab5B antibodies, revealing the presence of gold particles both on the food vacuole (FV) and the parasite plasma membrane (PPM, white arrows) in trophozoites and exclusively on parasite plasma membrane for late stages. P, parasite; r, rhoptry. Scale bars, 150 nm.Ezougou CN, Ben-Rached F, Moss DK, Lin JW, Black S, Knuepfer E, Green JL, Khan SM, Mukhopadhyay A, Janse CJ, Coppens I, Yera H, Holder AA, Langsley G. Plasmodium falciparum Rab5B Is an N-Terminally Myristoylated Rab GTPase That Is Targeted to the Parasite's Plasma and Food Vacuole Membranes. PLoS One. 2014 9(2):e87695.
See original on MMP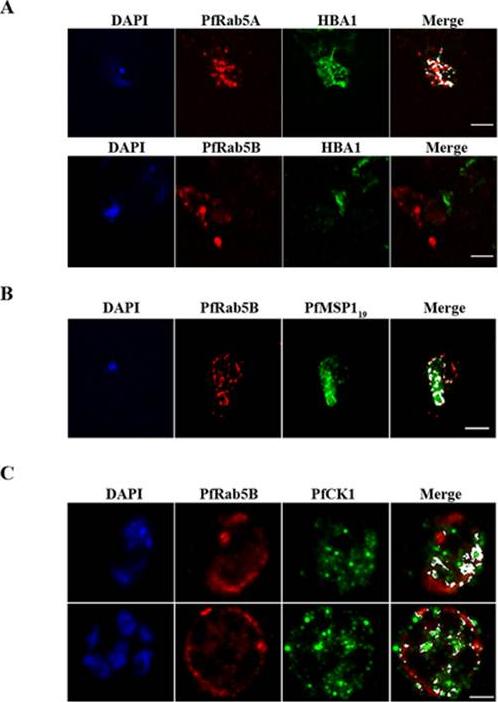
PfRab5B colocalises with PfMSP1 and PfCK1, but not with haemoglobin. (A) PfRab5A colocalises with haemoglobin (HBA1) containing vesicles unlike PfRab5B. (B) PfRab5B colocalises to differing degrees with the C-terminal 19 kDa fragment of PfMSP1 on structures close to the food vacuole and the parasite nucleus shown in blue by DAPI staining. (C) PfRab5B colocalises with PfCK1 on intracellular structures and at the parasite plasma membrane. Areas of colocalistaion are shown in white and used to calculate Pearson’s r coefficients. Scale bars, 2 mm.Ezougou CN, Ben-Rached F, Moss DK, Lin JW, Black S, Knuepfer E, Green JL, Khan SM, Mukhopadhyay A, Janse CJ, Coppens I, Yera H, Holder AA, Langsley G. Plasmodium falciparum Rab5B Is an N-Terminally Myristoylated Rab GTPase That Is Targeted to the Parasite's Plasma and Food Vacuole Membranes. PLoS One. 2014 9(2):e87695
See original on MMP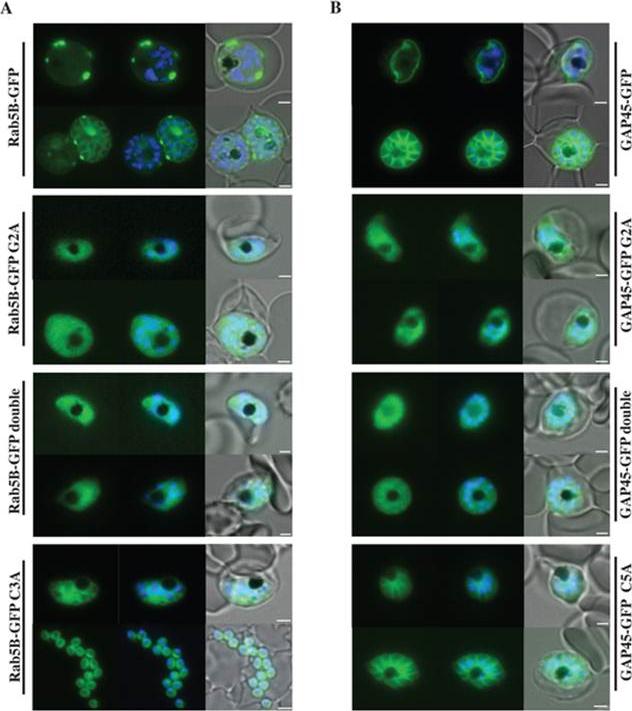
Localisation of PfRab5B-GFP fusion proteins in P. falciparum. Parasites were transfected with constructs expressing the N-terminal 28 amino acids of PfRab5B or 29 amino acids of PfGAP45 fused to GFP under a schizont stage-specific promoter (msp3 59UTR region). Localisation of PfRab5B28-GFP (A) and GAP4529-GFP fusions (B) as well as myristoylation (G2A) and palmitoylation-deficient fusions (C3A in PfRab5B28-GFP and C5A in GAP4529-GFP) were investigated. The first image in a series corresponds to GFP fluorescence, the second a merge of GFP fluorescence with nuclear DAPI stain, and the third a merge of GFP fluorescence, DAPI and bright field images. Size bars are 2 mm. GFP was observed at the plasma membrane (top right hand panel). In transgenic parasites that expressed GFP fused to G2A variants of both PfRab5B and GAP45 the association of GFP with the plasma membrane was lost and we observed a diffuse GFP-staining throughout the cytoplasm of the parasites .Ezougou CN, Ben-Rached F, Moss DK, Lin JW, Black S, Knuepfer E, Green JL, Khan SM, Mukhopadhyay A, Janse CJ, Coppens I, Yera H, Holder AA, Langsley G. Plasmodium falciparum Rab5B Is an N-Terminally Myristoylated Rab GTPase That Is Targeted to the Parasite's Plasma and Food Vacuole Membranes. PLoS One. 2014 9(2):e87695.
See original on MMP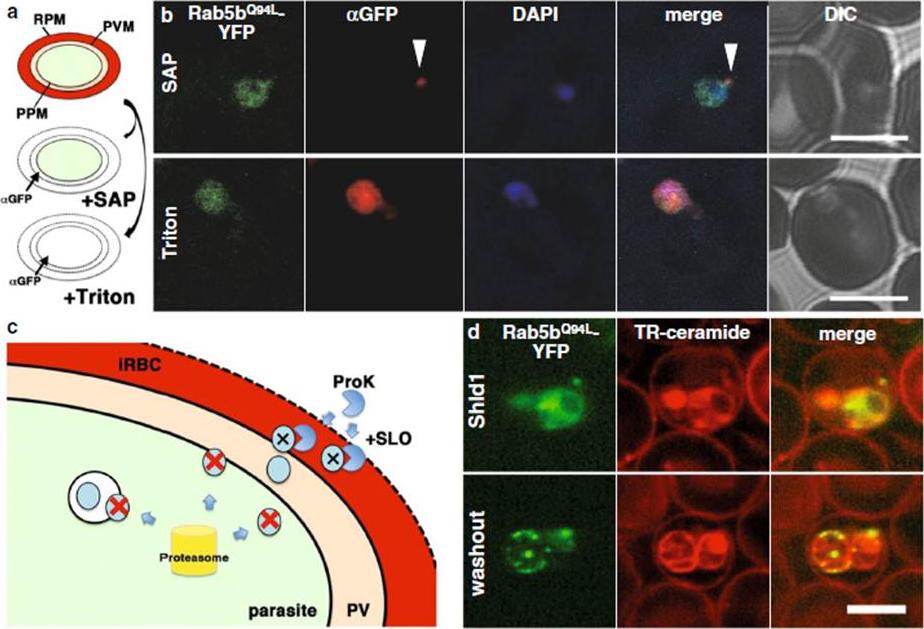
Transport of PfRab5bQ94L-YFP-DD to the cytoplasmic face of infected red blood cells on the TVN. a The selective permeabilization scheme. Saponin permeabilizes RBC plasma membrane (RPM) and PVM, which allows the detection of proteins localized to the outside of the parasite plasma membrane (PPM) or both sides of the PVM. Triton X-100 permeabilizes RPM, PVM, and PPM, allowing staining of the iRBCs, PV, and parasite cytosol with antibody. b Cells expressing PfRab5bQ94L-YFP-DD (green) were subjected to an antibody accessibility assay with anti-GFP antibody (red) prior to permeabilization with saponin (upper panel, SAP) or Triton X-100 (lower panel, Triton). After the saponin treatment, anti-GFP antibody labelled PfRab5bQ94L-YFP-DD secreted to the TVN (arrowhead), whereas PfRab5bQ94L-YFP-DD in the parasite cytosol was not labelled. Permeabilization with Triton X-100 allowed labelling of both the parasite cytosolic and TVN-localized PfRab5bQ94L-YFP-DD with anti-GFP antibody. c Schematic of the Shld1 washout assay and the protease accessibility assay. After removal of Shld1, cytosolic DD-tagged proteins are degraded (red cross and blue circle) by the parasite proteasome (yellow cylinder). DD-tagged proteins, inside of intracellular organelles or transported to the outside of the parasite (blue circular), are resistant to the proteasomal degradation. Since streptolysin O (SLO) permeabilizes RPM but not PVM, DD-tagged proteins in the RBC cytoplasm were selectively degraded by extracellular proteinase K (ProK) after permeabilization with SLO (black cross and blue circle). d Sub-cellular localization of PfRab5bQ94L-YFP-DD (green) after Shld1 stabilization for 24 h (upper panel, Shld1) and 2 h after Shld1 washout (lower panel, washout). After the removal of Shld1, the punctate signal of PfRab5bQ94L-YFP-DD localized to the TR-ceramide (red)-labelled parasite periphery. Bars 5 μm.Ebine K, Hirai M, Sakaguchi M, Yahata K, Kaneko O, Saito-Nakano Y. Plasmodium Rab5b is secreted to the cytoplasmic face of the tubovesicular network ininfected red blood cells together with N-acylated adenylate kinase 2. Malar J. 2016 15:323.
See original on MMP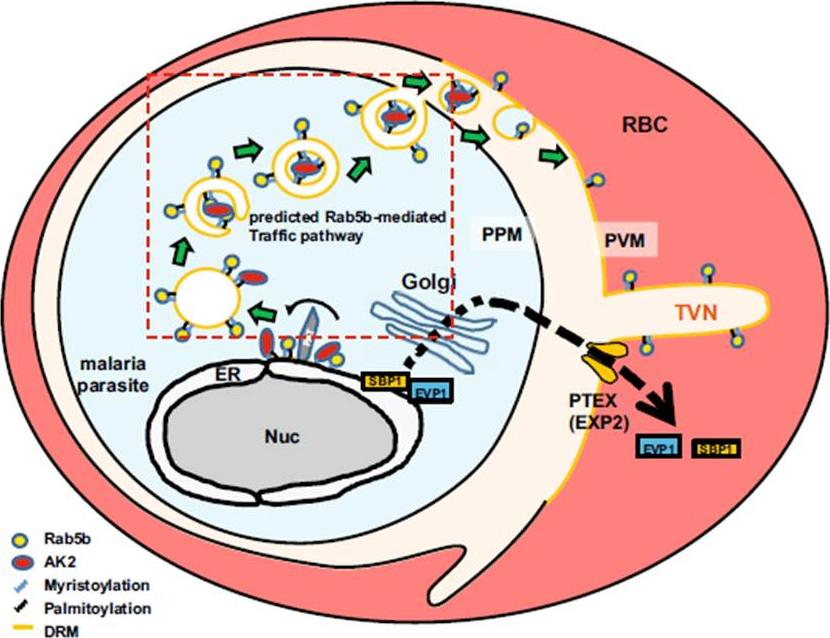
The hypothetical model of trafficking pathway involved in PfRab5b. N-acylated proteins, such as PfAK2, are recruited to novel compartment(s) adjacent to the ER, and packed into the multivesicular body or an autophagosome-like double membrane structure together with PfRab5b. The multivesicular body fuses with the PPM, and internal vesicles fuses with the PVM. Rab5b is clustered on the TVN. Nuc, nucleus; RBC, red blood cell; PTEX, Plasmodium translocon of exported proteins; PAT, palmitoyltransferase. EVP1 and SBP1 are exported proteins with and without thePlasmodium export element (PEXEL) signal, respectively .Ebine K, Hirai M, Sakaguchi M, Yahata K, Kaneko O, Saito-Nakano Y. Plasmodium Rab5b is secreted to the cytoplasmic face of the tubovesicular network in infected red blood cells together with N-acylated adenylate kinase 2. Malar J. 2016 15:323.
See original on MMP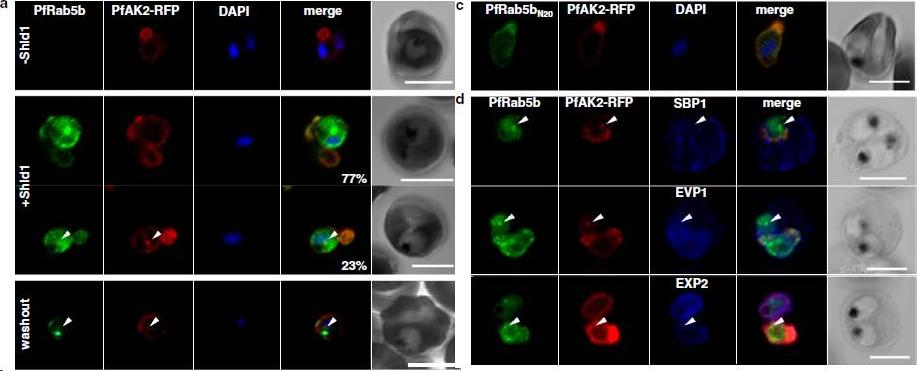
PfRab5b expression disturbed the transport of PfAK2 to the TVN. a In the absence of Shld1, PfAK2-RFP (red) localized to the TVN, while PfRab5b-YFP-DD signal was not detect (−Shld1, upper panel). After Shld1 stabilization for 48 h, PfRab5b-YFP-DD (green) and PfAK2-RFP co-localized in the TVN-like structures (+Shld1, middle-upper panel). In 77 % of PfRab5b-YFP-DD-positive parasites, PfAK2-RFP localized to the TVN, while in 23 % of PfRab5b-YFP-DD positive parasites, PfAK2-RFP signals also accumulated in the punctate compartment within the parasite (+Shld1, middle-lowerpanel, arrowhead). Co-localization of PfRab5b-YFP-DD and PfAK2-RFP at the punctate compartment remained even after removal of Shld1 (washout, lower panel, arrowhead). c No punctate localization of PfAK2-RFP was apparent with co-expression of PfRab5bN20-YFP-DD. d Effect of PfRab5b over-expression on the exported proteins PfSBP1, PfEVP1, and PfEXP2. In contrast to PfAK2-RFP, these proteins did not accumulate in the PfRab5b- and PfAK2-double positive punctate compartments (arrowhead).Ebine K, Hirai M, Sakaguchi M, Yahata K, Kaneko O, Saito-Nakano Y. Plasmodium Rab5b is secreted to the cytoplasmic face of the tubovesicular network in infected red blood cells together with N-acylated adenylate kinase 2. Malar J. 2016 15:323.
See original on MMP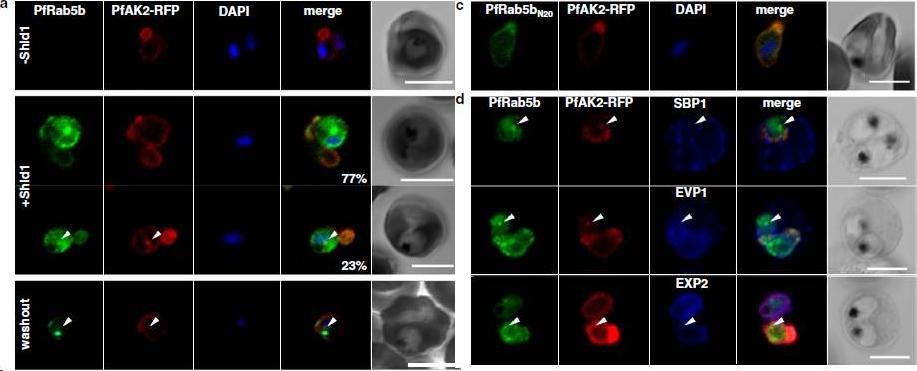
Localization of PfRab5bQ94L-YFP-DD to the TVN. a Time-lapse imaging of PfRab5bQ94L-YFP-DD (green) and TR-ceramide (red) fluorescence after stabilization with Shld1. PfRab5bQ94L-YFP-DD and TR-ceramide was co-localized to a rapidly moving compartment (arrowheads). b The pseudocolors of TR-ceramide at 0, 100, and 200 s were converted to red, green and blue, respectively, and then merged into a single frame. Several extended, mobile TVNs are shown in each color (arrowhead as an example), whereas a stable PVM is shown in white. (c) Cells expressing PfRab5bQ94L-YFP-DD (green) were stained with anti-PfTPx-1 antibody (red) and DAPI (blue). Cytoplasmic PfTPx-1 was not detected from the TVN, where PfRab5bQ94L-YFP-DD (arrowheads) localizes. Bars 5 μmץEbine K, Hirai M, Sakaguchi M, Yahata K, Kaneko O, Saito-Nakano Y. Plasmodium Rab5b is secreted to the cytoplasmic face of the tubovesicular network ininfected red blood cells together with N-acylated adenylate kinase 2. Malar J. 2016 15:323.
See original on MMP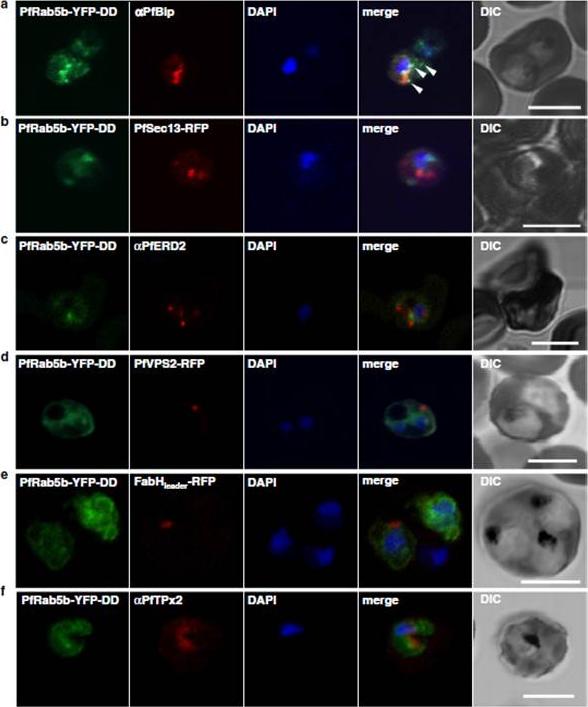
Localization of PfRab5b to a punctate compartment in the parasite cytoplasm. Triple staining with PfRab5b-YFP-DD (green), DAPI (blue) and one of the following markers (red): PfBip (a, ER), PfSec13-RFP (b, ER exit site), PfERD2 (c, Golgi), PfVPS2-RFP (d, putative multivesicular body/endosome), FabHleader-RFP (e, apicoplast), or PfTPx-2 (f, mitochondria) after 24 h incubation with Shld1. PfRab5b-YFP-DD localized adjacent to the Bip signal (arrowheads). Bars 5 μm.Ebine K, Hirai M, Sakaguchi M, Yahata K, Kaneko O, Saito-Nakano Y. Plasmodium Rab5b is secreted to the cytoplasmic face of the tubovesicular network in infected red blood cells together with N-acylated adenylate kinase 2. Malar J. 2016 15:323.
See original on MMPMore information
| PlasmoDB | PBANKA_1409100 |
| GeneDB | PBANKA_1409100 |
| Malaria Metabolic Pathways | Localisation images Pathways mapped to |
| Previous ID(s) | PB001554.02.0, PBANKA_140910 |
| Orthologs | PCHAS_1411000 , PF3D7_1310600 , PKNH_1411000 , PVP01_1411600 , PVX_122360 , PY17X_1410900 |
| Google Scholar | Search for all mentions of this gene |