PBANKA_0718800 small GTP-binding protein sar1, putative (SAR1)
Disruptability [+]
| Species | Disruptability | Reference | Submitter | |
|---|---|---|---|---|
| P. falciparum 3D7 |
Refractory |
18614010 | Theo Sanderson, Wellcome Trust Sanger Institute | |
| P. falciparum 3D7 |
Refractory |
USF piggyBac screen (Insert. mut.) | USF PiggyBac Screen | |
Mutant phenotypes [+]
None reported yet. Please press the '+' button above to add one.Imaging data (from Malaria Metabolic Pathways)
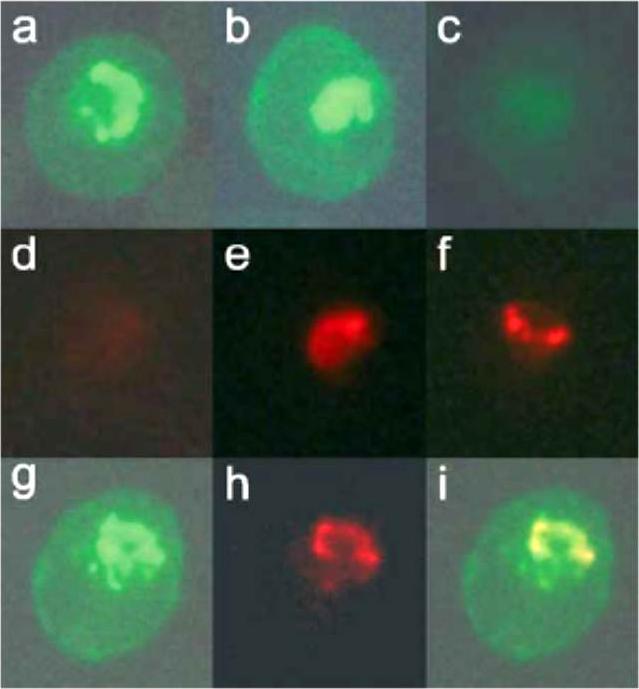
Dual-labeling experiment with anti-COPII components and mAb-134. P. falciparum-infected erythrocytes were fixed with glutaraldehyde and analyzed by immunofluorescence. Dual-labeling experiments were carried out with rabbit antisera against PfSec31p (panels a–f) or PfSar1p (panels g–i) and mAb-134. The samples were then incubated with a mixture of fluorescein-conjugated anti-rabbit IgG and rhodamine-conjugated anti-mouse IgG and examined under filters specific for fluorescein (panels a–c, g) or rhodamine (panels d–f, h). Shown are results of incubating with anti-PfSec31p alone (panels a and d), mAb-134 alone (panels c and f), or a mixture of both antibodies (panels b and e). No rhodamine signal is observed in the sample incubated with anti-PfSec31 (panel d) and no fluorescein signal is observed in the sample incubated with mAb-134 (panel c). Both antibodies predominantly recognize a compartment that is found on one side of the parasite and exhibit substantial overlap, suggesting that this subcellular compartment has some similarities to the endoplasmic reticulum or that this compartment represents a domain of the endoplasmic reticulum.. Samples were incubated with a mixture of rabbit anti-PfSar1p and mAb-134 followed by fluorescein and rhodamine-conjugated secondary antibodies. Shown are the results under filters specific for fluorescein (panel g) or rhodamine (panel h) or a merger between these two images (panel i).Cortes GT, Winograd E, Wiser MF. Characterization of proteins localized to a subcellular compartment associated with an alternate secretory pathway of the malaria parasite. Mol Biochem Parasitol. 2003 129:127-35. Copyright Elsevier
See original on MMP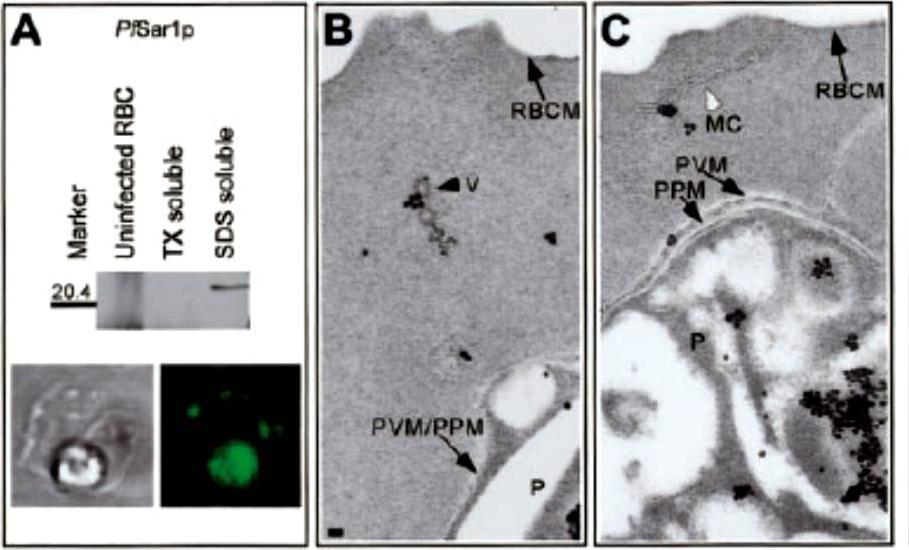
Pf Sar1p is associated with coated vesicles and Maurer clefts. (A) Western blot analysis of uninfected and IRBCs incubated with AlF demonstrated that Pf Sar1p was an approximate 23-kDa Triton X-100–insoluble, SDS-soluble protein that was absent in uninfected RBCs. IFA showed Pf Sar1p localized to the parasite compartment and structures in the RBC cytosol. Immunoelectron microscopy revealed Pf Sar1p associated with (B) coated vesicles (V), black arrowhead, and (C) Maurer clefts (MC), white arrowhead, within the RBC cytosol. Scale bar =70 nm. Taraschi TF, O'Donnell M, Martinez S, Schneider T, Trelka D, Fowler VM, Tilley L, Moriyama Y. Generation of an erythrocyte vesicle transport system by Plasmodium falciparum malaria parasites. Blood. 2003 102:3420-3426.
See original on MMP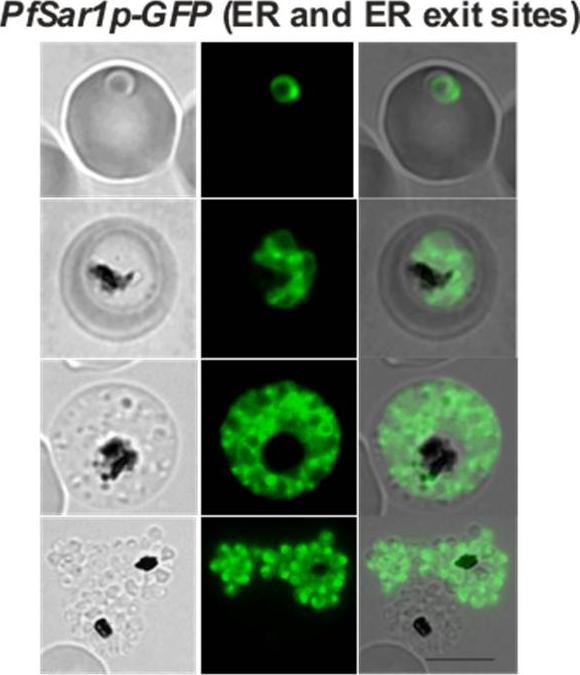
Confocal fluorescence microscopy images of transfected 3D7 P. falciparum-infected RBCs expressing a GFP chimera of PfSar1p located at the ER and at ER exit sites. Bright puncta of fluorescence are observed in addition to the ER pattern. DIC image, the GFP fluorescence signal and an overlay of PfSar1p-GFP transfectant. Scale bar = 5 µm.Adisa A, Frankland S, Rug M, Jackson K, Maier AG, Walsh P, Lithgow T, Klonis N, Gilson PR, Cowman AF, Tilley L. Re-assessing the locations of components of the classical vesicle-mediated trafficking machinery in transfected Plasmodium falciparum. Int J Parasitol. 2007 37(10):1127-41. Copyright Elsevier 2009; PMID:
See original on MMP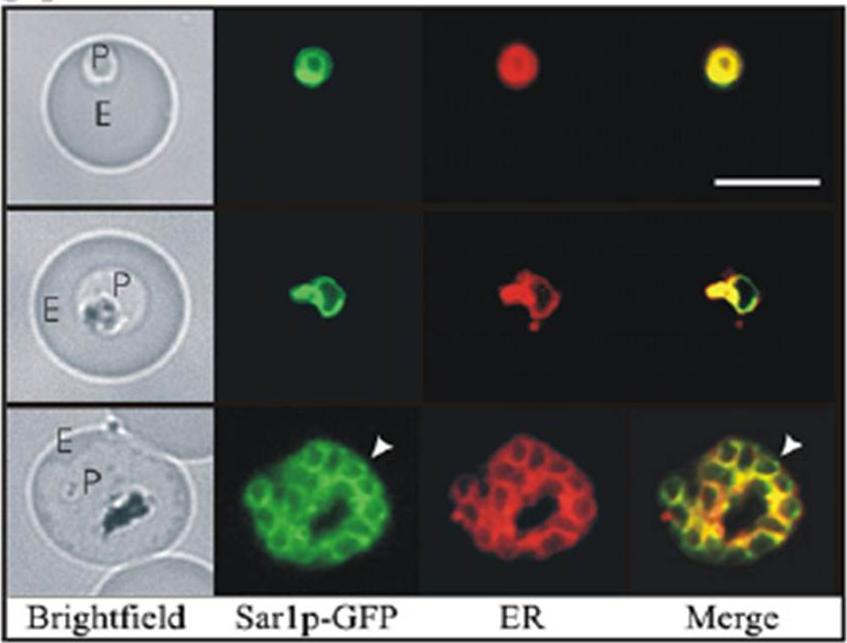
Co-labelling of the PfSar1p chimeras in transfected parasites with the endoplasmic reticulum (ER) probe ER TrackerTMTilley L, McFadden G, Cowman A, Klonis N. Illuminating Plasmodium falciparum-infected red blood cells. Trends Parasitol. 2007 23:268-77. Copyright Elsevier 2009;
See original on MMP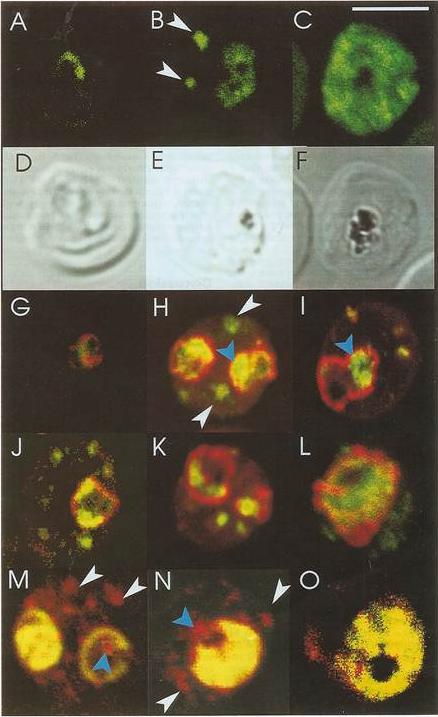
Confocal immunofluorescence of PfSar1p. Panels A-C: Parasitized erythrocyes (D10 strain) were labeled with affinity purified anti=PfSar1p antiserum followed by fluorescein-conjugated anti-rabbit antiserum (green). Panels D-F: Transmission images of panels A-C. Panels G-L: Dual labeling of affinity-purified rabbit anti-PfSar1p antiserum followed by fluorescein-conjugated anti-rabbit antiserum (green) and a murine monoclonal antibody recognizing the parasitophorous vacuole-located protein, Exp-1, followed by rhodamine-conjugated anti-mouse antiserum (red). Sar1p in the trophozoite is clearly located in the erythrocyte cytosol (G-K) and in the schizont it occupies most of it (L). Panels M-O: Dual labeling of affinity-purified rabbit anti-PfSar1p antiserum followed by rhodamine-conjugated anti-mouse antiserum (red) and a biotin-conjugated anti-PfERC antiserum followed by fluorescein-conjugated anti-rabbit antiserum (green). PfERC and PfSar1p labeling patterns do not completely coincide, indicating that PfSar1p is not confined to the ER. An optical slice was collected through the center of the parasite by confocal microscopy. Some PfSar1p-containing structures in the erythrocyte cytosol are marked with white arrowheads. Some PfSar1p-containing structures in the parasite cytosol are marked with blue arrowheads. The bar in panel C corresponds to 5 mm.Albano FR, Berman A, La Greca N, Hibbs AR, Wickham M, Foley M, Tilley L. A homologue of Sar1p localises to a novel trafficking pathway in malaria-infected erythrocytes. Eur J Cell Biol. 1999 78:453-462. Copyright Elsevier 2010.
See original on MMP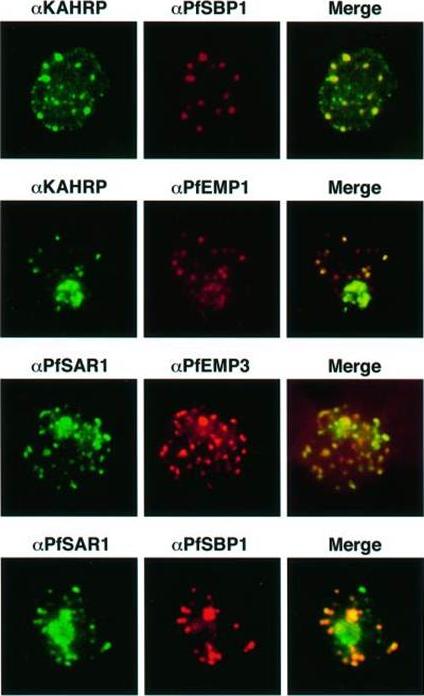
Transient co-localization of KAHRP, PfSBP1, PfEMP1, PfEMP3 and PfSAR1p to Maurer’s clefts in ring stage P.falciparum-infected erythrocytes. First row: co-localization of KAHRP (green) with PfSBP1 (red). Second row: co-localization of KAHRP (green) with PfEMP1 (red). Third row: co-localization of PfSAR1p (green) with PfEMP3 (red). Fourth row: co-localization of PfSAR1p (green) with PfSBP1 (red). The third window in each row represents the merging of the red and green channels. Yellow areas represent regions of co-localization.Wickham ME, Rug M, Ralph SA, Klonis N, McFadden GI, Tilley L, Cowman AF. Trafficking and assembly of the cytoadherence complex in Plasmodium falciparum-infected human erythrocytes. EMBO J. 2001 20(20):5636-49.
See original on MMP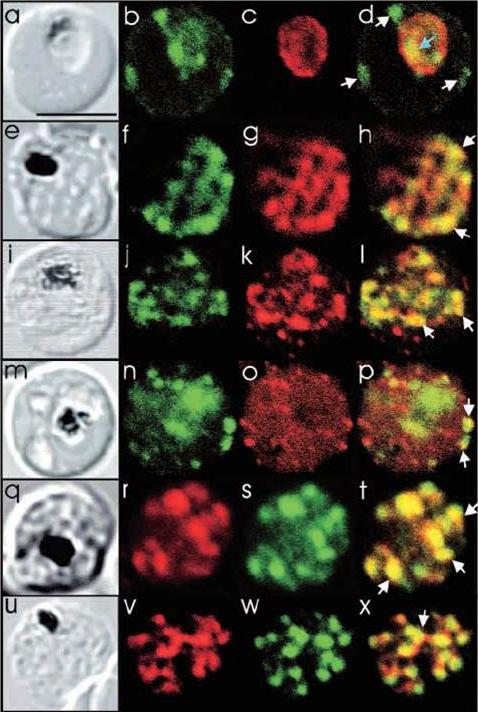
Comparison of the location of PfSec31p with that of other parasite antigens in P. falciparum-infected erythrocytes. (a-d) Asynchronous infected erythrocytes (K1 strain) were probed with affinity-purified rabbit anti-PfSec31(WD) antiserum followed by a fluorescein-conjugated anti-rabbit IgG (b; green fluorescence) and a murine monoclonal antibody recognising the PV-located protein Exp1 followed by rhodamine conjugated anti-mouse IgG (c; red fluorescence). An optical slice was collected through the centre of the parasite by confocal microscopy. Some PfSec31p-containing structures in the erythrocyte cytosol are marked with white arrowheads and a PfSec31p-containing structure in the parasite cytosol is marked with a blue arrowhead (d). (a) A transmission image of (b-d). (e-l) Asynchronous infected erythrocytes (K1 strain) were probed with mouse anti-PfSec31 (WD) antiserum followed by a fluorescein-conjugated anti-mouse IgG (green fluorescence) and a rabbit anti-PfSar1p antiserum followed by Alexa-Fluor-568-conjugated anti-rabbit IgG (red fluorescence). Optical slices were collected near the surface of the parasitised erythrocytes. Some PfSec31p- and PfSar1p-containing structures are marked with arrowheads. (m-p) Asynchronous infected erythrocytes were probed with affinity-purified rabbit anti-PfSec31( WD) antiserum followed by fluorescein-conjugated anti-rabbit IgG (green fluorescence) and a murine monoclonal antibody recognising PfEMP3, followed by Alexa-Fluor-568-conjugated anti-mouse IgG (red florescence). An optical slice was collected near the surface of the parasitised erythrocyte. Two structures in which there is partial overlap of PfSec31p and PfEMP3 in the erythrocyte cytosol are marked with arrowheads. (q-x) Asynchronous infected erythrocytes were probed with a mouse anti-PfSec31(WD) antiserum followed by Alexa-Fluor-568-conjugated anti-mouse IgG (red fluorescence) and an affinity-purified rabbit anti-PfEMP1 antibody followed by fluorescein-conjugated anti-rabbit IgG (green florescence). Optical slices were collected near the surface of the parasitised erythrocytes. Some PfSec31p- and PfEMP1-containing structures in the erythrocyte cytosol are marked with arrowheads. Bar in (a), 5 mm.Adisa A, Albano FR, Reeder J, Foley M, Tilley L. Evidence for a role for a Plasmodium falciparum homologue of Sec31p in the export of proteins to the surface of malaria parasite-infected erythrocytes. J Cell Sci. 2001 114(Pt 18):3377-86.
See original on MMPMore information
| PlasmoDB | PBANKA_0718800 |
| GeneDB | PBANKA_0718800 |
| Malaria Metabolic Pathways | Localisation images Pathways mapped to |
| Previous ID(s) | PB000267.00.0, PBANKA_071880 |
| Orthologs | PCHAS_0727900 , PF3D7_0416800 , PKNH_0509200 , PVP01_0526200 , PVX_089930 , PY17X_0718900 |
| Google Scholar | Search for all mentions of this gene |