PY17X_0610200 zinc finger transcription factor, putative (KROX1)
Disruptability [+]
| Species | Disruptability | Reference | Submitter | |
|---|---|---|---|---|
| P. berghei ANKA |
Refractory |
PlasmoGEM (Barseq) | PlasmoGEM | |
| P. berghei ANKA |
Possible |
RMgm-4316 | Imported from RMgmDB | |
| P. falciparum 3D7 |
Possible |
USF piggyBac screen (Insert. mut.) | USF PiggyBac Screen | |
Mutant phenotypes [+]
| Species | Stage | Phenotype | Reference | Submitter |
|---|---|---|---|---|
| P. berghei ANKA | Asexual |
Difference from wild-type |
RMgm-4316
Normal growth/multiplication characteristics of blood stages. Mice infected with Zfp-KO parasites survived longer (2x) than mice infected with wild type parasites |
Imported from RMgmDB |
| P. berghei ANKA | Gametocyte |
No difference |
RMgm-4316 | Imported from RMgmDB |
| P. berghei ANKA | Ookinete |
No difference |
RMgm-4316 | Imported from RMgmDB |
| P. berghei ANKA | Oocyst |
Difference from wild-type |
RMgm-4316
Oocyst numbers in mosquitoes infected with KO parasites were 98% to 99% lower than those seen with mosquitoes infected with wild type parasites |
Imported from RMgmDB |
Imaging data (from Malaria Metabolic Pathways)
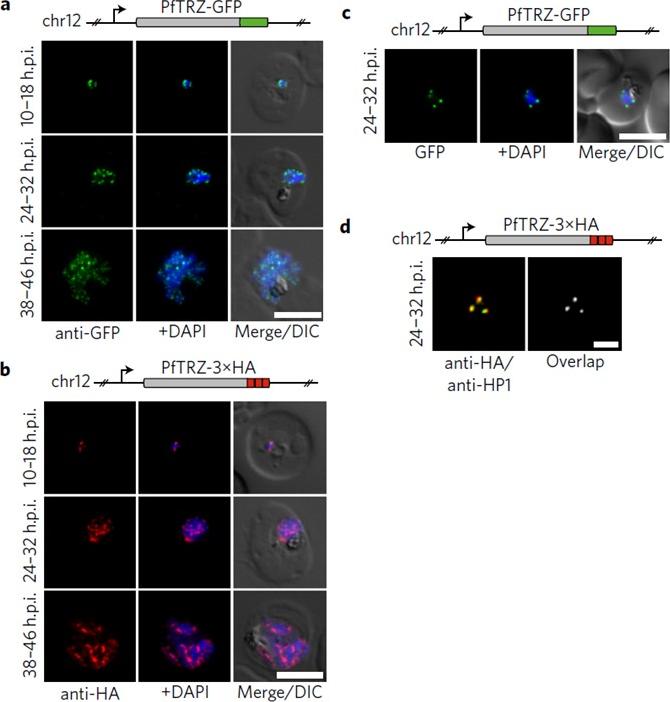
Genome-wide binding profile of PfTRZ. a,b, Localization of endogenous PfTRZ-GFP (a) and PfTRZ-3×HA (b), detected by IFA in three different IDC stages. h.p.i., hours post-invasion. Scale bars, 5 μm. Results are representative of three biological replicate experiments. c, Live imaging of endogenous PfTRZ-GFP. Scale bar, 5 μm. Results are representative of three biological replicate experiments. d, Co-localization of PfTRZ-3×HA and PfHP1 by confocal laser-scanning microscopy. The Pearson co-localization map visualizes overlapping signals (right). Scale bar, 2 μm. PfTRZ-GFP and PfTRZ-3×HA localized to distinct foci at the nuclear periphery throughout the intra-erythrocytic developmental cell cycle. The number of PfTRZ foci and total PfTRZ expression levels increased in schizonts (a,b). Confocal laser-scanning microscopy confirmed the exclusive co-localization of PfTRZ with PfHP1 at chromosome-end clusters.Bertschi NL, Toenhake CG, Zou A, Niederwieser I, Henderson R, Moes S, Jenoe P, Parkinson J, Bartfai R, Voss TS. Malaria parasites possess a telomere repeat-binding protein that shares ancestry with transcription factor IIIA. Nat Microbiol. 2017 2:17033. PMID: 28288093
See original on MMP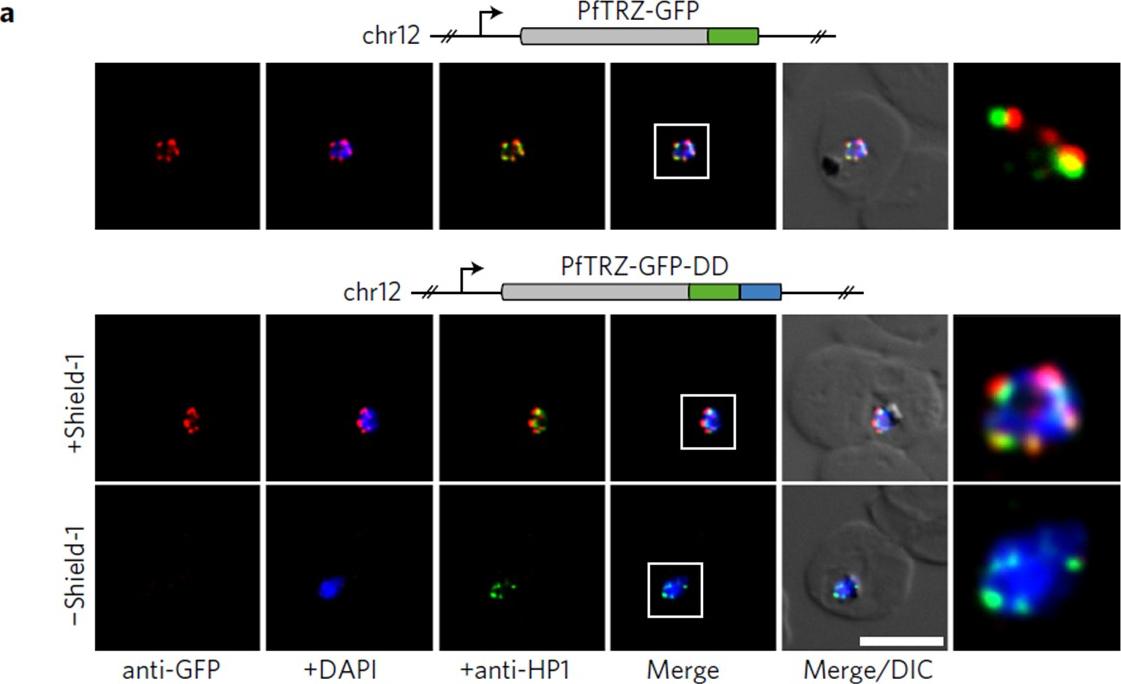
PfTRZ is required for proliferation and telomere length homeostasis. a, Co-localization of PfTRZ and PfHP1 in 3D7/TRZGFP, 3D7/TRZGFP-ON(+Shield-1) and 3D7/TRZGFP-OFF (−Shield-1) trophozoites. White frames refer to the magnified view in the rightmost images. Scale bar, 5 μm. Results are representative of three biological replicate experiments. PfTRZ-GFP-DD displayed the expected perinuclear expression pattern in 3D7/TRZGFP-ON parasites, but was barely detectable in parasites cultured in the absence of Shield-1 (3D7/TRZGFP-OFF).Bertschi NL, Toenhake CG, Zou A, Niederwieser I, Henderson R, Moes S, Jenoe P, Parkinson J, Bartfai R, Voss TS. Malaria parasites possess a telomere repeat-binding protein that shares ancestry with transcription factor IIIA. Nat Microbiol. 2017 2:17033.
See original on MMP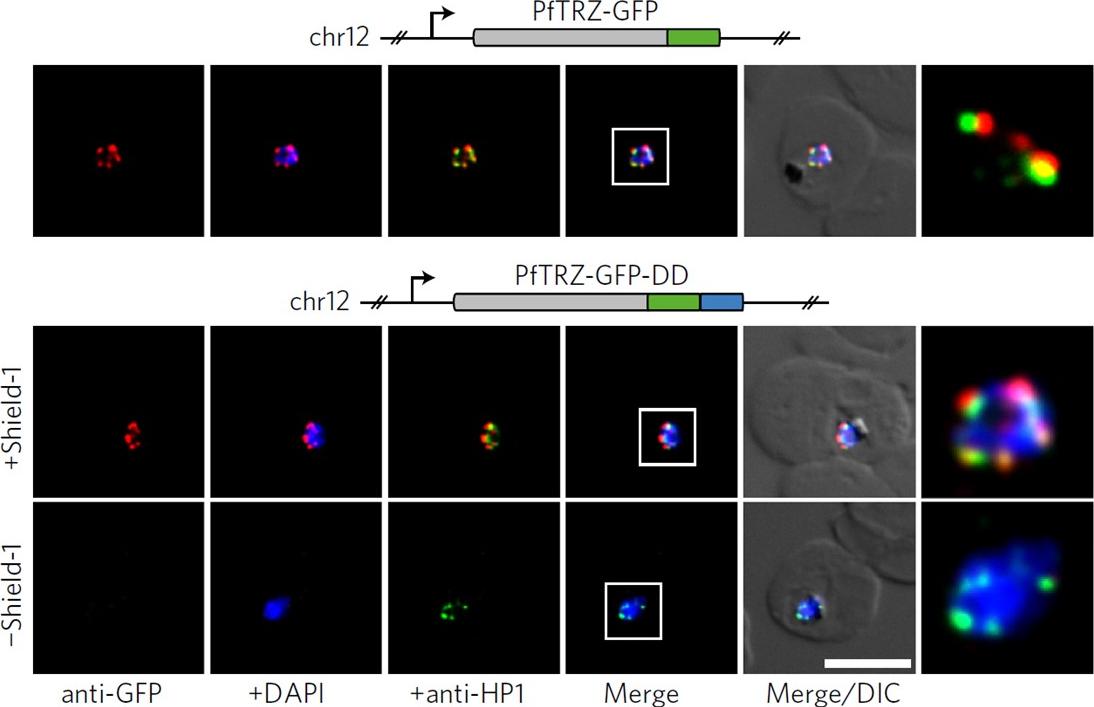
PfTRZ is required for proliferation and telomere length homeostasis. a, Co-localization of PfTRZ and PfHP1 in 3D7/TRZGFP, 3D7/TRZGFP-ON(+Shield-1) and 3D7/TRZGFP-OFF (−Shield-1) trophozoites. White frames refer to the magnified view in the rightmost images. Scale bar, 5 μm. Results are representative of three biological replicate experiments. PfTRZ-GFP-DD displayed the expected perinuclear expression pattern in 3D7/TRZGFP-ON parasites, but was barely detectable in parasites cultured in the absence of Shield-1 (3D7/TRZGFP-OFF).Bertschi NL, Toenhake CG, Zou A, Niederwieser I, Henderson R, Moes S, Jenoe P, Parkinson J, Bartfai R, Voss TS. Malaria parasites possess a telomere repeat-binding protein that shares ancestry with transcription factor IIIA. Nat Microbiol. 2017 2:17033. PMID:28288093
See original on MMP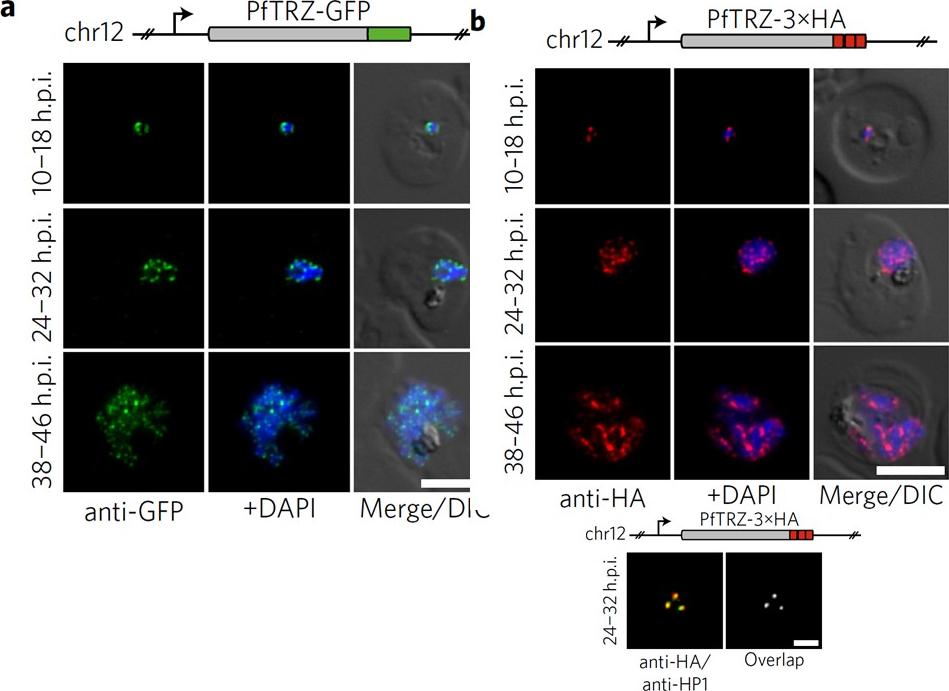
Genome-wide binding profile of PfTRZ. a,b, Localization of endogenous PfTRZ-GFP (a) and PfTRZ-3×HA (b), detected by IFA in three different IDC stages. h.p.i., hours post-invasion. Scale bars, 5 μm. Results are representative of three biological replicate experiments. transgenic parasites expressing PfTRZ-GFP (3D7/TRZGFP) or PfTRZ-3×HA (3D7/TRZHA) (HA, haemagglutinin) from the endogenous locus (Supplementary Fig. 2). As expected, both PfTRZ-GFP and PfTRZ-3×HA localized to distinct foci at the nuclear periphery throughout the intra-erythrocytic developmental cell cycle (IDC) (Fig. 2a–c). Consistent with the consecutive rounds of genome duplication and nuclear division during schizogony, the number of PfTRZ foci and total PfTRZ expression levels increased in schizonts.Bertschi NL, Toenhake CG, Zou A, Niederwieser I, Henderson R, Moes S, Jenoe P, Parkinson J, Bartfai R, Voss TS. Malaria parasites possess a telomere repeat-binding protein that shares ancestry with transcription factor IIIA. Nat Microbiol. 2017 2:17033.
See original on MMP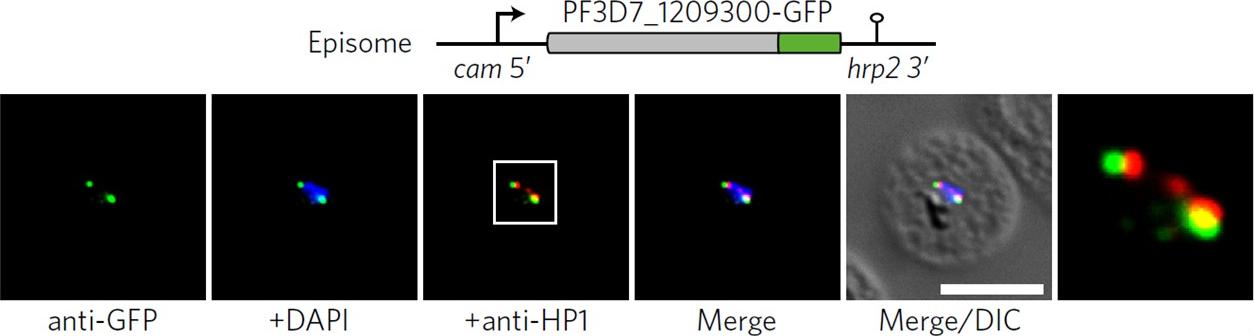
Co-localization of ectopic PF3D7_1209300-GFP and PfHP1 in trophozoites. The white frame refers to the magnified view in the rightmost image. DIC, differential interference contrast. Scale bar, 5 μm. Results are representative of three biological replicate experiments. IFAs demonstrated that an ectopically expressed green fluorescent protein (GFP)-tagged version of this factor localized to distinct spots at the nuclear periphery, in close proximity to the chromosome end marker PfHP1 (HP1, heterochromatin protein 1.Bertschi NL, Toenhake CG, Zou A, Niederwieser I, Henderson R, Moes S, Jenoe P, Parkinson J, Bartfai R, Voss TS. Malaria parasites possess a telomere repeat-binding protein that shares ancestry with transcription factor IIIA. Nat Microbiol. 2017 2:17033.
See original on MMPMore information
| PlasmoDB | PY17X_0610200 |
| GeneDB | PY17X_0610200 |
| Malaria Metabolic Pathways | Localisation images Pathways mapped to |
| Previous ID(s) | null |
| Orthologs | PBANKA_0607700 , PCHAS_0609500 , PF3D7_1209300 , PKNH_1309200 , PVP01_1308400 , PVX_084495 |
| Google Scholar | Search for all mentions of this gene |