PF3D7_1116000 rhoptry neck protein 4 (RON4)
Disruptability [+]
| Species | Disruptability | Reference | Submitter | |
|---|---|---|---|---|
| P. berghei ANKA |
Refractory |
RMgm-686 | Imported from RMgmDB | |
| P. berghei ANKA |
Refractory |
PlasmoGEM (Barseq) | PlasmoGEM | |
Mutant phenotypes [+]
None reported yet. Please press the '+' button above to add one.Imaging data (from Malaria Metabolic Pathways)
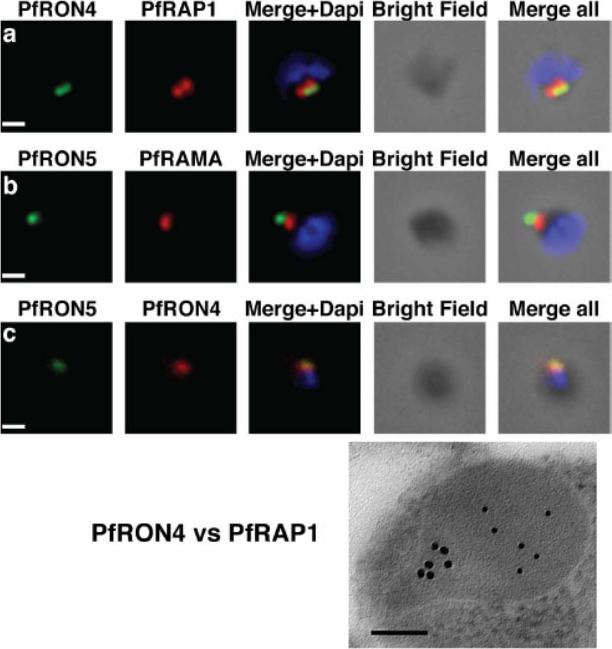
The PfRON4 and PfRON5 proteins localize to the rhoptry neck. A, Antibodies raised against PfRON4 and PfRON5 recognize specific protein products in schizont parasite extracts. B, IFAs using the anti-PfRON antibodies reveal staining of the apical tip of free merozoite, in close apposition with the rhoptry bulb markers PfRAP1 and PfRAMA, suggesting a rhoptry neck localization. Scale bar: 0.2μm C, Immunoelectron microscopy confirms that PfRON 4 localizes to the rhoptry neck. Scale bar: 0.1μmRichard D, Macraild CA, Riglar DT, Chan JA, Foley M, Baum J, Ralph SA, Norton RS, Cowman AF. Interaction between Plasmodium falciparum apical membrane antigen 1 and the Rhoptry neck protein complex defines a key step in the erythrocyte invasion process of malaria parasites. J Biol Chem. 2010 285:14815-22
See original on MMP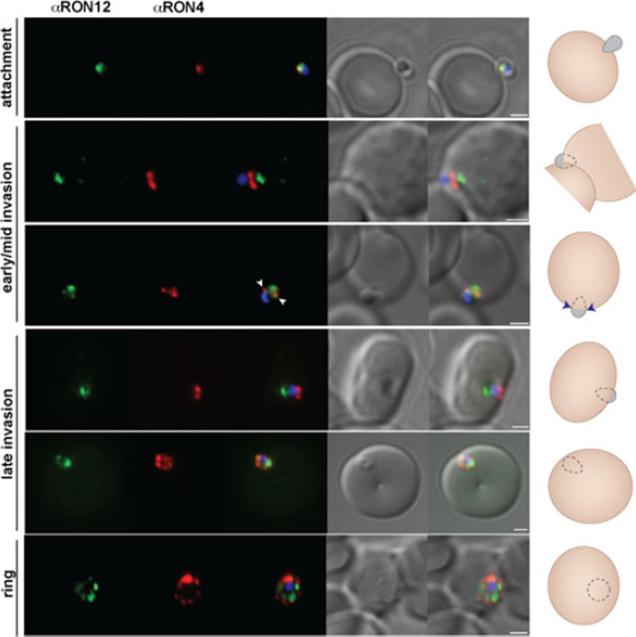
RON12 is predominantly secreted late into the nascent PV. Immunofluorescence and DIC images of merozoites fixed during invasionof erythrocytes. From left to right anti-RON12 (green), anti-RON4 depicting the moving junction (red), overlay of both with nuclear stain DAPI, DIC image and overlay of all images. Cartoon schematic is shown on the right of each panel. Arrowheads depict location of moving junction. Size bars equal 1 μm.Knuepfer E, Suleyman O, Dluzewski AR, Straschil U, O'Keeffe AH, Ogun SA, Green JL, Grainger M, Tewari R, Holder AA. RON12, a novel Plasmodium-specific rhoptry neck protein important for parasite proliferation. Cell Microbiol. 2013 Aug 12. [Epub ahead of print]
See original on MMP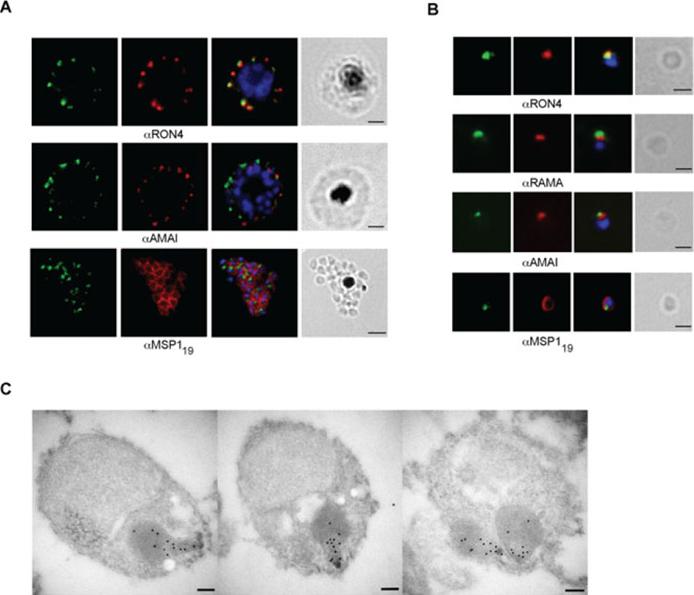
RON12 is located within the rhoptry neck.. A and B. Immunofluorescence and bright-field images of schizonts (A) and merozoites (B). Images from left to right show anti-RON12 labelling (green) followed by microneme (anti-AMA1), rhoptry (neck: anti-RON4; body: anti-RAMA) and surface (anti-MSP119) specific antibodies in red, overlay of both with DAPI-stained nuclei and the bright-field image. Size bars in (A) equal 2 μm and in (B) 1 μm. C. Immuno-electron microscopy of isolated merozoites. Localization of RON12 in the rhoptry neck of three different merozoites is shown. Size bars equal 100 nm.Knuepfer E, Suleyman O, Dluzewski AR, Straschil U, O'Keeffe AH, Ogun SA, Green JL, Grainger M, Tewari R, Holder AA. RON12, a novel Plasmodium-specific rhoptry neck protein important for parasite proliferation. Cell Microbiol. 2013 Aug 12. [Epub ahead of print]
See original on MMP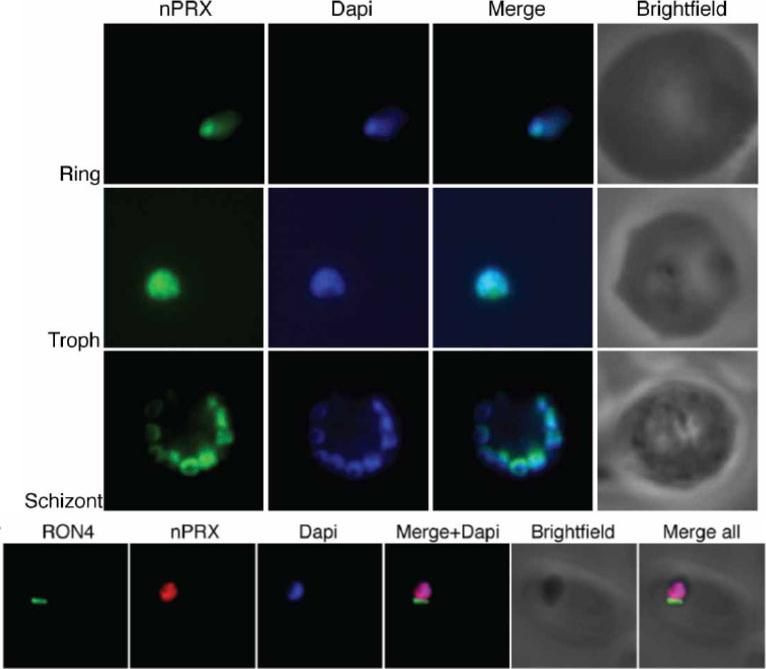
PfnPrx is a nuclear protein. Top) Immuno-fluorescence using the mouse monoclonal anti-PfnPrx antibody demonstrates localisation of PfnPrx in the nucleus throughout the erythrocytic stage. Bottom) PfnPrx does not localise to the tight junction of merozoites in the process of invasion. Immunofluorescence with mouse monoclonal anti-nPrx and rabbit anti-RON4 antibody shows that PfnPrx is restricted to the nucleus in parasites invading a red blood cell. Richard D, Bartfai R, Volz J, Ralph SA, Muller S, Stunnenberg HG, Cowman AF. A genome-wide chromatin-associated nuclear from the malaria parasite Plasmodium falciparum. J Biol Chem. 2011 286(13):11746-55
See original on MMP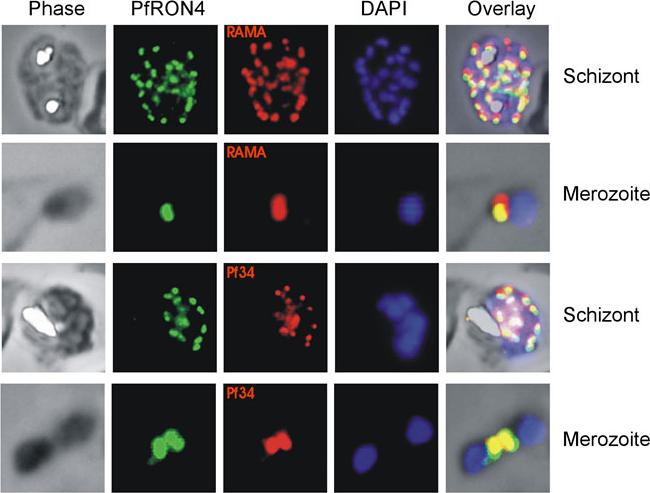
Co-localisation IFAs were performed on mature schizont-infected red blood cells and ruptured merozoites using our anti-PfRON4 antisera and antisera against the rhoptry marker proteins RAMA and Pf34 PFD0955w. Co-localisation of PfRON4 with both RAMA MAL7P1.208 and Pf34 is observed, with marginally better co-localisation observed with Pf34, a reported rhoptry neck protein.Morahan BJ, Sallmann GB, Huestis R, Dubljevic V, Waller KL. Plasmodium falciparum: Genetic and immunogenic characterisation of the rhoptry neck protein PfRON4. Exp Parasitol. 2009 122(4):280-8.
See original on MMP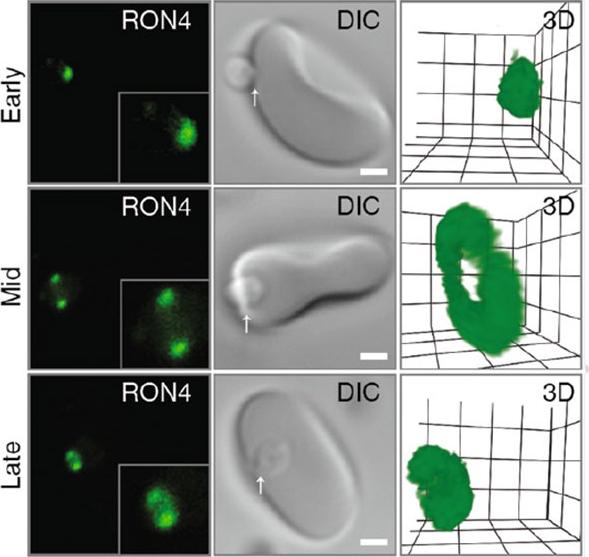
Confocal microscopy of early, mid and late merozoite invasion, showing (left ) single slice immunofluorescence of the tight junction labeled with anti-RON4, (middle) accompanying DIC images of the same invasion event and (right) a 3D projection of the anti-RON4 tight junction labeling. Arrows mark the relative position of tight junction labeling. Scale bar = 1 mm. Inset shows 2× zoom of merozoite. In 3D projections, grid = 0.5 mm and gamma settings have been changed for display purposes.Riglar DT, Baum J. Static and Dynamic Imaging of Erythrocyte Invasion and Early Intra-erythrocytic Development in Plasmodium falciparum. Methods Mol Biol. 2013;923:269-80.
See original on MMP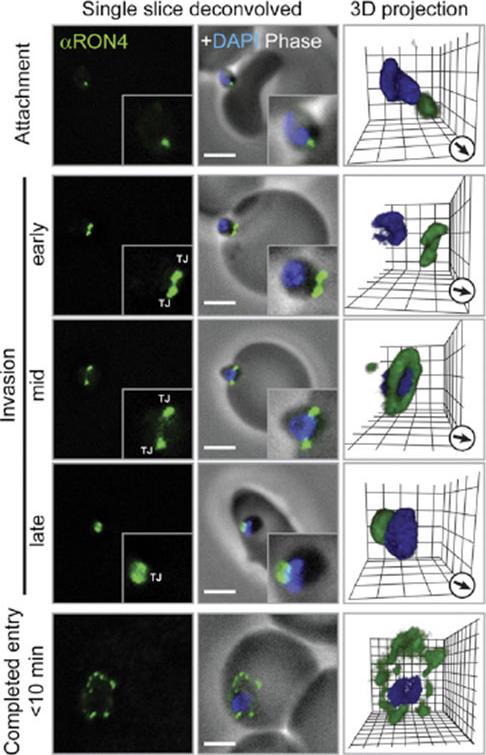
Immunofluorescence Microscopy of P. falciparum Merozoite Invasion Time courses of invasion by wide-field immunofluoresence assay (IFA) microscopy with deconvolution (single slice or three-dimensional reconstruction) labeled with PfRON4. For TEM numbering, see the main text. The IFA scale bar represents 2.0 mm. In 3D images, gamma settings were altered, and the grid represents 0.5 mm intervals. DG, dense granules; Mi, micronemes; N, nucleus; PV, parasitophorous vacuole; R, rhoptries; TJ, tight junction.Riglar DT, Richard D, Wilson DW, Boyle MJ, Dekiwadia C, Turnbull L, Angrisano F, Marapana DS, Rogers KL, Whitchurch CB, Beeson JG, Cowman AF, Ralph SA, Baum J. Super-resolution dissection of coordinated events during malaria parasite invasion of the human erythrocyte. Cell Host Microbe. 2011 9:9-20.
See original on MMP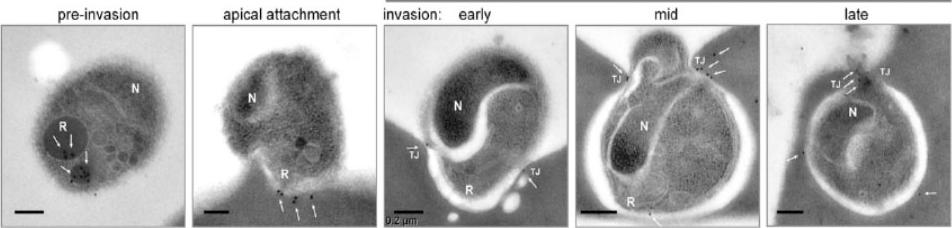
Representative transmission electron microscopy with immunogold labeling (white arrows) of PfRON4 (the scale bar represents 0.2 mm). PfRON4 immunogold labeling was retained within the rhoptry neck preinvasion but significantly moved, in the majority to the cytosolic side of the erythrocyte membrane at the time of attachment (white arrows). Once inside, labeling could be seen to associate directly with the junction.Riglar DT, Richard D, Wilson DW, Boyle MJ, Dekiwadia C, Turnbull L, Angrisano F, Marapana DS, Rogers KL, Whitchurch CB, Beeson JG, Cowman AF, Ralph SA, Baum J. Super-resolution dissection of coordinated events during malaria parasite invasion of the human erythrocyte. Cell Host Microbe. 2011 9:9-20
See original on MMPPfRON4 localizes to the rhoptry necks. Immuno-electron microscopy with MAb 24C6 and protein A-gold shows specific labeling in the rhoptry necks of P. falciparum schizonts. Infected human erythrocytes were embedded in LRWhite and stained with MAb 24C6. The enlarged area shows a longitudinally sectioned rhoptry with PfRON4 labeling restricted to the narrow apical end of the rhoptries in a maturing merozoite (arrows).Alexander DL, Arastu-Kapur S, Dubremetz JF, Boothroyd JC. Plasmodium falciparum AMA1 binds a rhoptry neck protein homologous to TgRON4, a component of the moving junction in Toxoplasma gondii. Eukaryot Cell. 2006 5:1169-73.
See original on MMP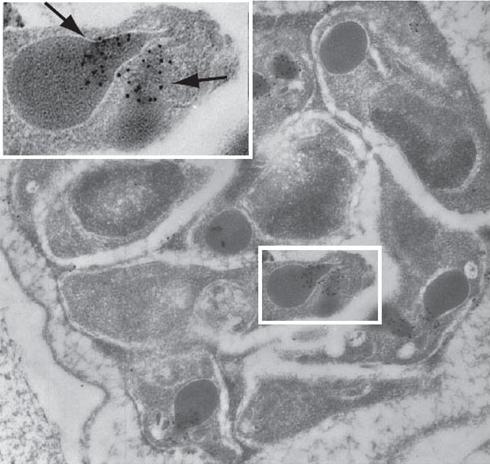
PfRON4 localizes to the rhoptry necks. Immuno-electron microscopy with MAb 24C6 and protein A-gold shows specific labeling in the rhoptry necks of P. falciparum schizonts. Infected human erythrocytes were embedded in LRWhite and stained with MAb 24C6. The enlarged area shows a longitudinally sectioned rhoptry with PfRON4 labeling restricted to the narrow apical end of the rhoptries in a maturing merozoite (arrows).Alexander DL, Arastu-Kapur S, Dubremetz JF, Boothroyd JC. Plasmodium falciparum AMA1 binds a rhoptry neck protein homologous to TgRON4, a component of the moving junction in Toxoplasma gondii. Eukaryot Cell. 2006 5:1169-73.
See original on MMP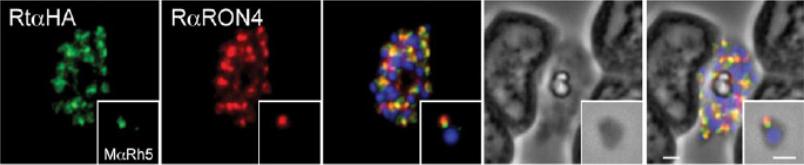
Immunofluorescence and phase contrast images of late schizonts or free merozoites (insets) to colocalise PfRh5 with RON4. Each panel from left top right corresponds to anti-HA antibodies (to detect PhRh5), rabbit anti-RON4, overlay of each with DAPI nuclear stain, phase contrast and overlay of all images. Insets show individual merozoites. Scale bars = 1 mM. RON4 associates with PfRh5 which localises to the rhoptries in merozoites and follows the tight junction during the process of erythrocyte invasion.Baum J, Chen L, Healer J, Lopaticki S, Boyle M, Triglia T, Ehlgen F, Ralph SA, Beeson JG, Cowman AF. Reticulocyte-binding protein homologue 5 - An essential adhesin involved in invasion of human erythrocytes by Plasmodium falciparum. Int J Parasitol. 2009 39:371-80.
See original on MMP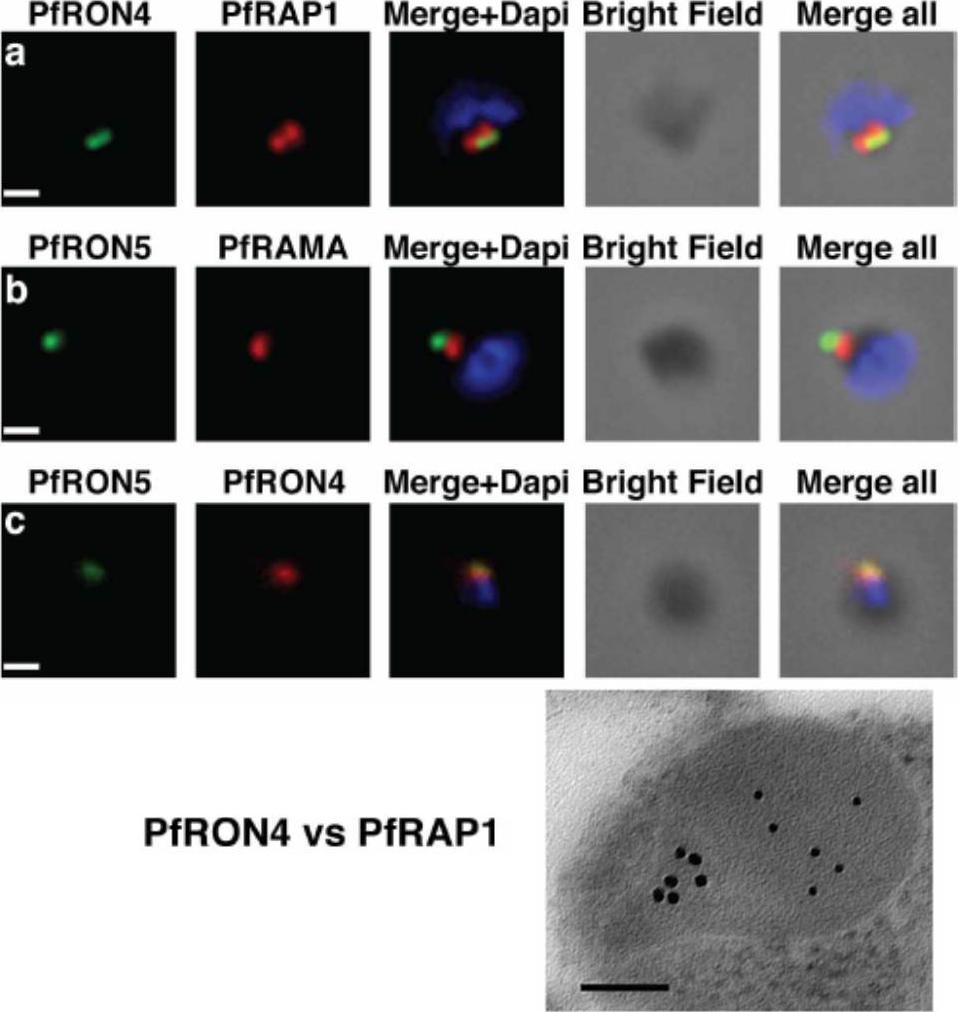
The PfRON4 and PfRON5 proteins localize to the rhoptry neck. A, Antibodies raised against PfRON4 and PfRON5 recognize specific protein products in schizont parasite extracts. B, IFAs using the anti-PfRON antibodies reveal staining of the apical tip of free merozoite, in close apposition with the rhoptry bulb markers PfRAP1 and PfRAMA, suggesting a rhoptry neck localization. Scale bar: 0.2μm C, Immunoelectron microscopy confirms that PfRON 4 localizes to the rhoptry neck. Scale bar: 0.1μmRichard D, Macraild CA, Riglar DT, Chan JA, Foley M, Baum J, Ralph SA, Norton RS, Cowman AF. Interaction between Plasmodium falciparum apical membrane antigen 1 and the Rhoptry neck protein complex defines a key step in the erythrocyte invasion process of malaria parasites. J Biol Chem. 2010 285:14815-22
See original on MMP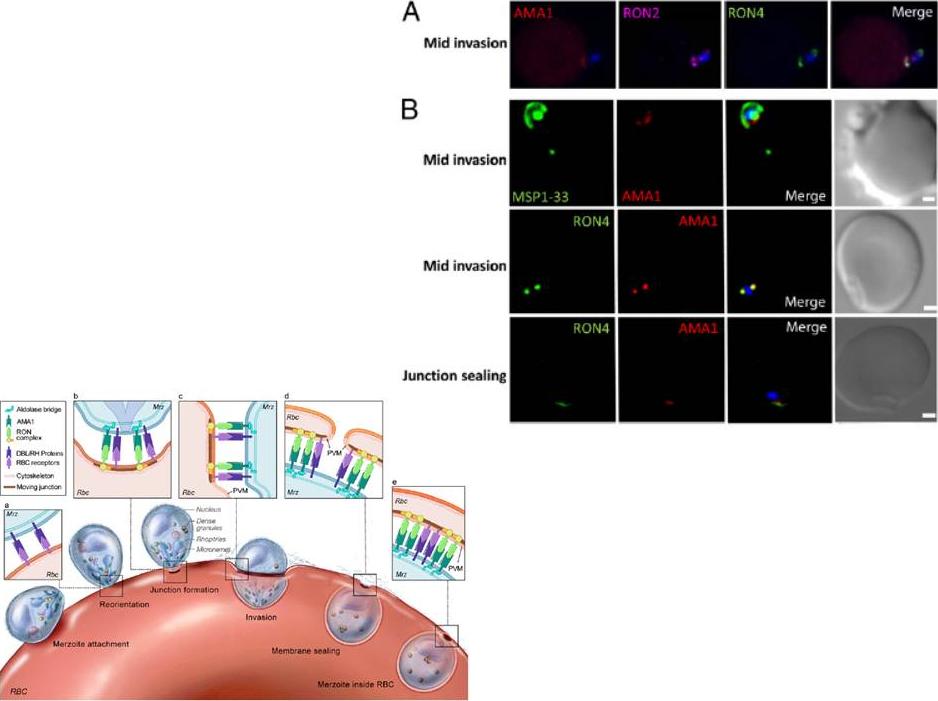
Localization of Pf AMA1, RON2, and RON4 at the moving junction of invading merozoites. Confocal microscopy images of merozoite invading RBC are shown. (A) Pf AMA1, RON2, and RON4 colocalize during invasion. (B) AMA1 is present at the moving junction (Top) with RON4 colocalizing with AMA1 at the junction (Middle). RON4 marks the sealing of the junction after invasion (Bottom). Scale bars represent 1μM.Schematic model of the steps involved in Pf merozoite (Mrz) invasion.Srinivasan P, Beatty WL, Diouf A, Herrera R, Ambroggio X, Moch JK, Tyler JS, Narum DL, Pierce SK, Boothroyd JC, Haynes JD, Miller LH. Binding of Plasmodium merozoite proteins RON2 and AMA1 triggers commitment to invasion. Proc Natl Acad Sci U S A. 2011 108(32):13275-80
See original on MMP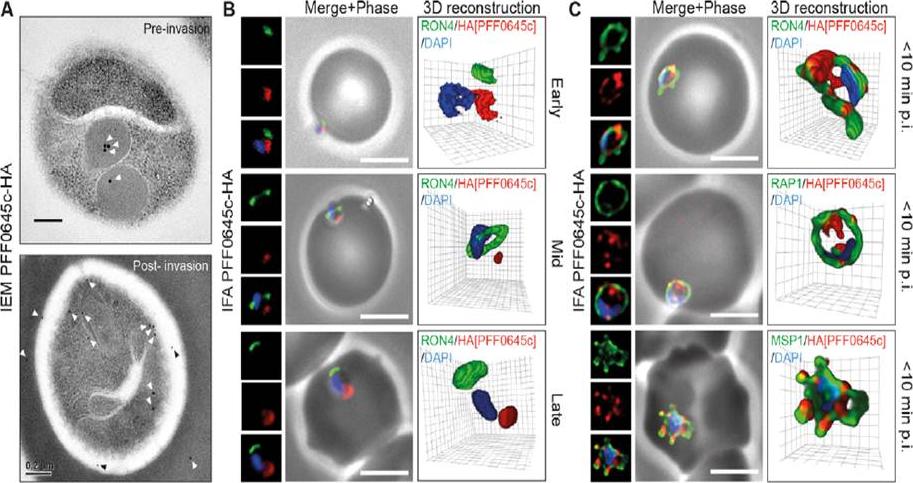
PFF0645c is released from the rhoptries only after completion of merozoite invasion. (A) IEM of PFF0645c-HA merozoites pre and post-erythrocyte invasion labeled with immunogold anti-HA (white arrows). Scale bar = 0.2 mm. (B) Widefield 3D imaging of PFF0645c-HA merozoites labeled with anti-HA, anti-PfRON4 and DAPI showing early, mid and late invasion events. Scale bar = 5 mm. 3D reconstruction with 0.2 mm grid intervals. (C) Widefield 3D imaging of PFF0645c-HA early rings (,10 min post-invasion) labeled with anti-HA, anti-PfRON4 (,PVM) anti-RAP1 (PV) or anti-MSP1 (plasma membrane) and DAPI. Scale bar = 5 mm. 3D reconstruction with 0.2 mm grid intervals.Zuccala ES, Gout AM, Dekiwadia C, Marapana DS, Angrisano F, Turnbull L, Riglar DT, Rogers KL, Whitchurch CB, Ralph SA, Speed TP, Baum J. Subcompartmentalisation of Proteins in the Rhoptries Correlates with Ordered Events of Erythrocyte Invasion by the Blood Stage Malaria Parasite. PLoS One. 2012;7(9):e46160.
See original on MMP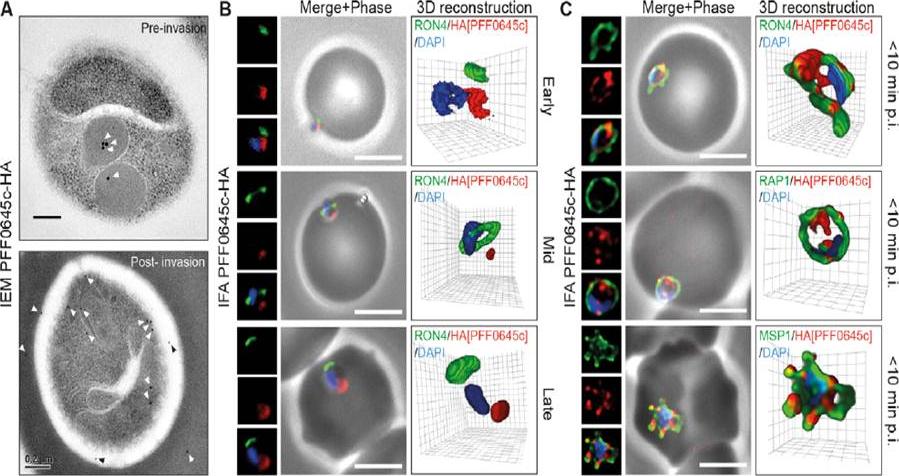
PFF0645c is released from the rhoptries only after completion of merozoite invasion. (A) IEM of PFF0645c-HA merozoites pre and post-erythrocyte invasion labeled with immunogold anti-HA (white arrows). Scale bar = 0.2 mm. (B) Widefield 3D imaging of PFF0645c-HA merozoites labeled with anti-HA, anti-PfRON4 and DAPI showing early, mid and late invasion events. Scale bar = 5 mm. 3D reconstruction with 0.2 mm grid intervals. (C) Widefield 3D imaging of PFF0645c-HA early rings (,10 min post-invasion) labeled with anti-HA, anti-PfRON4 (,PVM) anti-RAP1 (PV) or anti-MSP1 (plasma membrane) and DAPI. Scale bar = 5 mm. 3D reconstruction with 0.2 mm grid intervals.Zuccala ES, Gout AM, Dekiwadia C, Marapana DS, Angrisano F, Turnbull L, Riglar DT, Rogers KL, Whitchurch CB, Ralph SA, Speed TP, Baum J. Subcompartmentalisation of Proteins in the Rhoptries Correlates with Ordered Events of Erythrocyte Invasion by the Blood Stage Malaria Parasite. PLoS One. 2012;7(9):e46160.
See original on MMP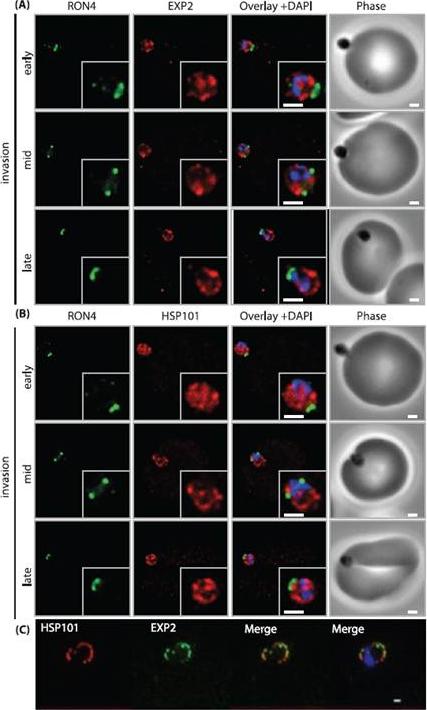
PTEX components are carried into the newly infected erythrocyte and are present at the PVM throughout intraerythrocytic development. (A and B) Time-course of merozoite invasion by widefield immunofluorescence microscopy with deconvolution (single slice shown) labelled for (A) tight junction component RON4 and PTEX component EXP2 or (B) RON4 and PTEX component HSP101. Both PTEX components are carried by the invading merozoite into the erythrocyte. Scale bar: 1μm. (C) HSP101 and EXP2 co-localise to distinct puncta at the PVM during later ring stage development. Scale bar: 1μm. Bullen HE, Charnaud SC, Kalanon M, Riglar DT, Dekiwadia C, Kangwanrangsan N, Torii M, Tsuboi T, Baum J, Ralph SA, Cowman AF, de Koning-Ward TF, Crabb BS, Gilson PR. Biosynthesis, localisation and macromolecular arrangement of the Plasmodium falciparum translocon of exported proteins; PTEX. J Biol Chem. 2012 287(11):7871-84
See original on MMP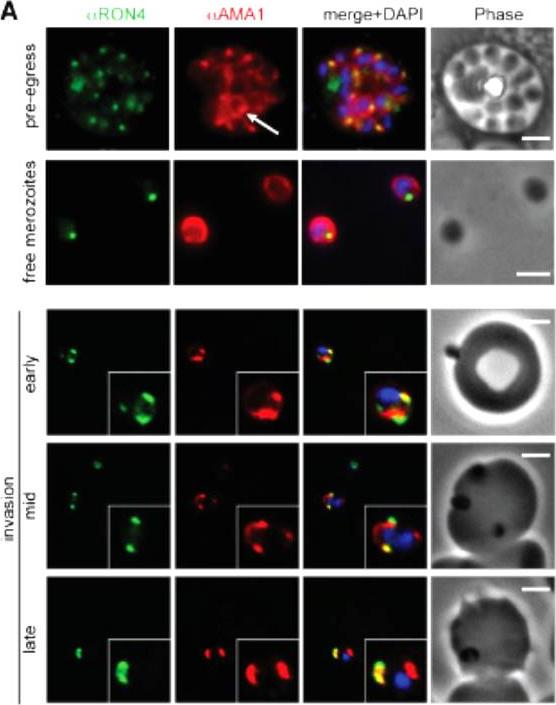
Wide field IFA time course of invasion from pre-egress through to invasion using anti-PfAMA1/PfRON4. Pre-egress and free merozoites show standard wide-field image. Invasion images show IFA with deconvolution (single slice). Scale bar = 2.0μm. In parasites fixed prior to or immediately after egress from E64-treated schizonts (ETS) a significant proportion of PfAMA1 had already been secreted onto the merozoite surface. Conventional fluorescence imaging of midinvasion merozoites revealed that PfAMA1 labeling localized directly within the plane of the ring of PfRON4 fluorescence. A proportion of PfAMA1 was also variably localized to the merozoite surface and unreleased micronemes.Riglar DT, Richard D, Wilson DW, Boyle MJ, Dekiwadia C, Turnbull L, Angrisano F, Marapana DS, Rogers KL, Whitchurch CB, Beeson JG, Cowman AF, Ralph SA, Baum J. Super-resolution dissection of coordinated events during malaria parasite invasion of the human erythrocyte. Cell Host Microbe. 2011 9:9-20.
See original on MMP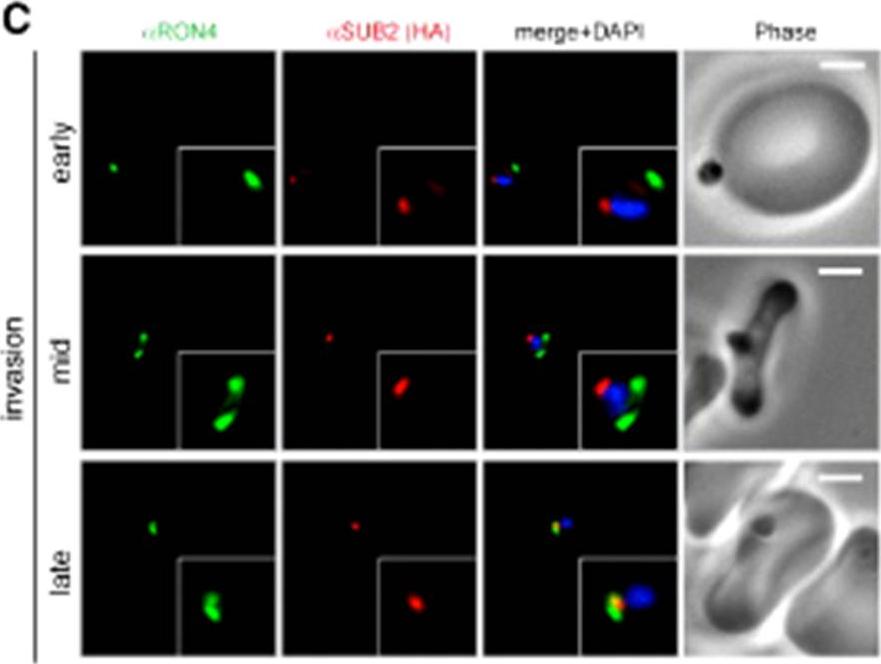
Wide field IFA time course of invasion using anti-HA(PfSUB2HA)/PfRON4. PfSUB2, is a micronemal protease, sheddase, responsible for MSP1 release. Scale bar = 2.0 μm. In parasites at early stages of invasion, the majority of PfSUB2 had reached the posterior pole of the merozoite (PfSUB2 early). Although not colocalized in the same parasite, the lack of contiguity between shed MSP1 and PfSUB2 sheddase, each in comparison with PfRON4 (PfSUB2 mid), suggests that MSP1 may be processed by PfSUB2 early in invasion but shed only during passage through the junction. In the latter stages of invasion, PfSUB2 remained at the posterior pole (SUB2 late).Riglar DT, Richard D, Wilson DW, Boyle MJ, Dekiwadia C, Turnbull L, Angrisano F, Marapana DS, Rogers KL, Whitchurch CB, Beeson JG, Cowman AF, Ralph SA, Baum J. Super-resolution dissection of coordinated events during malaria parasite invasion of the human erythrocyte. Cell Host Microbe. 2011 9:9-20.
See original on MMP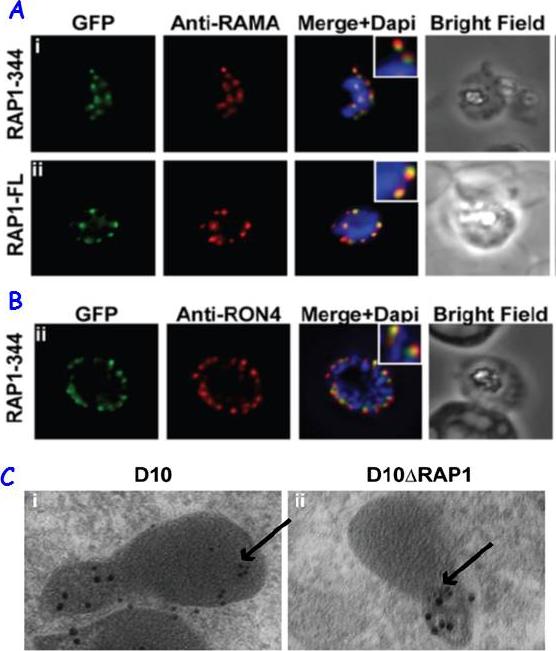
RAP1 contains a bipartite rhoptry signal. A RAP1-344 and RAP1-FL GFP fusions. Both constructs show a punctate fluorescence pattern characteristic of rhoptry localisation. For the RAP1-FL construct, GFP signal overlaps with RAMA. In contrast, the RAP1-344GFP chimera only partially overlaps with RAMA, suggesting rhoptry neck localisation. B. For the RAP1-344 construct, GFP co-localises with the rhoptry neck marker PfRON4. C. Immunoelectron microscopy demonstrates that truncated RAP1 (10 nm beads) in D10DRAP1 parasites is localised in the rhoptry neck, whereas full-length RAP1 in D10 (wild-type) parasites is localised in the rhoptry bulb. PfRON4 PF11_0168 (15 nm beads) is localised in the rhoptry neck in both parasite lines.Richard D, Kats LM, Langer C, Black CG, Mitri K, Boddey JA, Cowman AF, Coppel RL. Identification of Rhoptry Trafficking Determinants and Evidence for a Novel Sorting Mechanism in the Malaria Parasite Plasmodium falciparum. PLoS Pathog. 2009 5(3):e1000328.
See original on MMP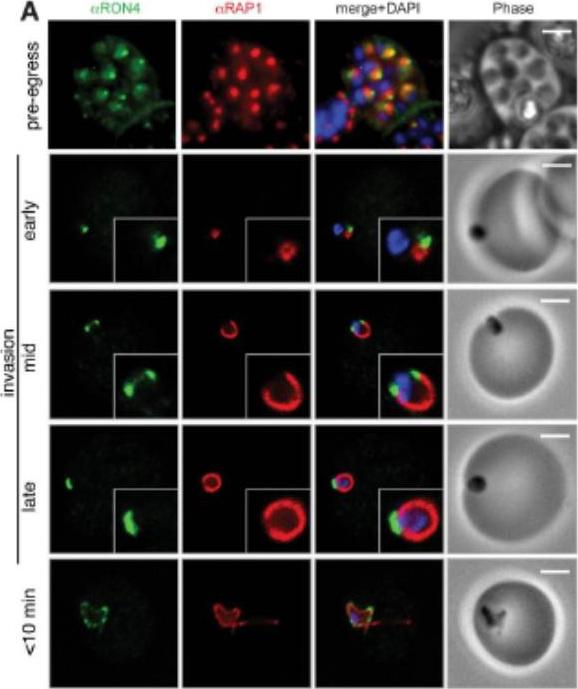
Wide field IFA time course of invasion using A) anti-RAP1/PfRON4. Pre-egress shows standard wide-field image. Invasion images show IFA with deconvolution (single slice). Before invasion commencement, RAP1 clearly remained within the merozoite apex (RAP1 early) posterior to the rhoptry neck as defined by PfRON4. Midinvasion merozoites revealed that RAP1 labeling had moved to an anterior position beginning immediately adjacent to the junction and tracking the exterior of the merozoite as it entered (RAP1 mid). These observations show clearly RAP1 transition pre-, mid-, and postinvasion.Riglar DT, Richard D, Wilson DW, Boyle MJ, Dekiwadia C, Turnbull L, Angrisano F, Marapana DS, Rogers KL, Whitchurch CB, Beeson JG, Cowman AF, Ralph SA, Baum J. Super-resolution dissection of coordinated events during malaria parasite invasion of the human erythrocyte. Cell Host Microbe. 2011 9:9-20.
See original on MMP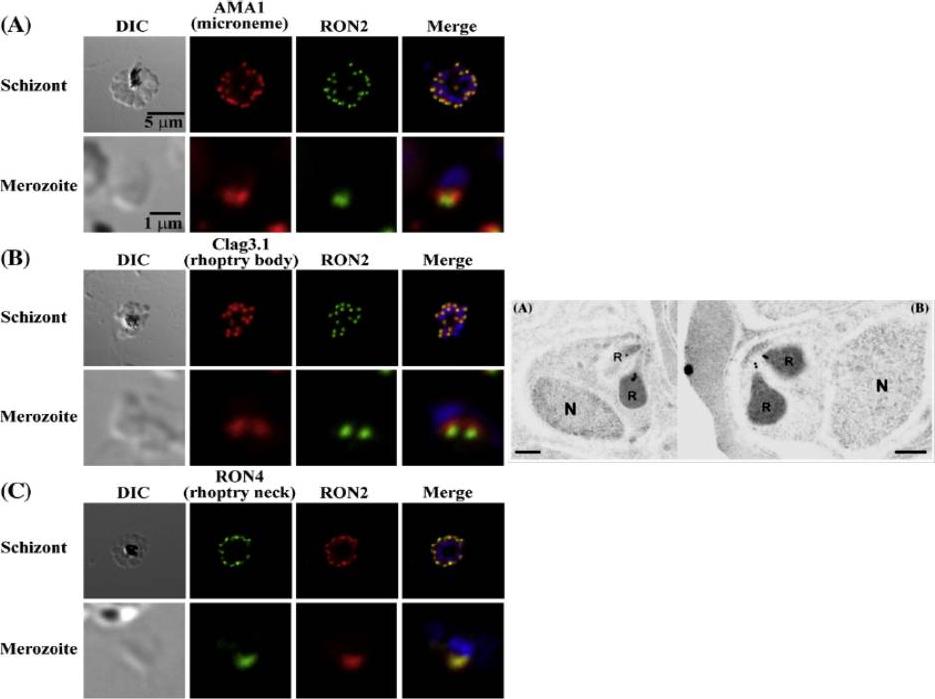
Left: PfRON2 is expressed at the apical end of Plasmodium merozoites. Schizont infected erythrocytes and merozoites were dual-labeled with antisera against PfRON2 and PfAMA1 PF11_0344 (A), PfClag3.1 PFC0110w (B), or PfRON4 PF11_0168 (C). Merged images are shown in the right panels. All segmented schizonts and merozoites are positive for PfRON2. Nuclei are counterstained with DAPI. Colocalization of PfRON2 with PfRON4 (rhoptry neck marker) was observed but neither colocalized with PfClag3.1 (rhoptry body marker) nor PfAMA1 (microneme marker). Right: Longitudinally sectioned merozoites in schizont-infected erythrocytes were labeled with anti-PfRON2 serum followed by secondary Ab conjugated with gold particles. Gold particles were restricted to the narrow neck portion of the rhoptries (R). Two different images are shown (A and B). N indicates nucleus. Bars=200 nm. Cao J, Kaneko O, Thongkukiatkul A, Tachibana M, Otsuki H, Gao Q, Tsuboi T, Torii M. Rhoptry neck protein RON2 forms a complex with microneme protein AMA1 in Plasmodium falciparum merozoites. Parasitol Int. 2009 58:29-35.
See original on MMP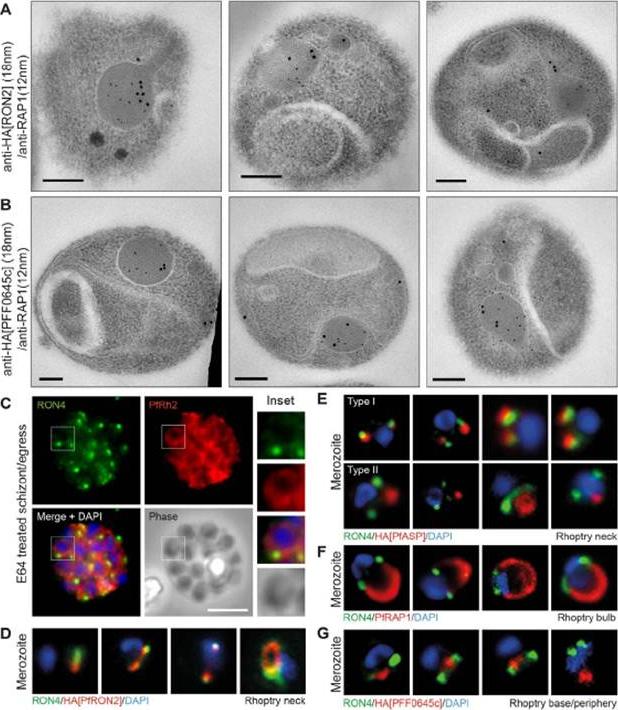
Spatial localisation of different rhoptry proteins before and during merozoite invasion. (A) IEM of free PfRON2-HA merozoites (pre-invasion) dual labeled with immunogold anti-HA (18 nm) and rhoptry bulb marker RAP1 (12 nm). Scale bar = 0.2 mm. (B) IEM of free PFF0645c-HA merozoites (pre-invasion) dual labeled with immunogold anti-HA (18 nm) and rhoptry bulb marker RAP1 (12 nm). Scale bar = 0.2 mm. (C) Widefield IFA of E64-treated schizonts (to prevent egress labeled with anti-PfRh2, anti-PfRON4 and DAPI. Scale bar = 5 mm. (D–G) Independent replicate imaging of merozoites from (D) PfRON2-HA, (E) PfASP-HA (two classes of distribution seen), (F) RAP1 and (G) PFF0645c-HA mid-way through invasion colabeled with anti-PfRON4 and DAPI. RON2-HA is located anterior to the bulb marker RAP1. PFF0645c had a more posterior or contiguous localisation to RAP1, frequently located towards the peripheral regions of the rhoptry.Zuccala ES, Gout AM, Dekiwadia C, Marapana DS, Angrisano F, Turnbull L, Riglar DT, Rogers KL, Whitchurch CB, Ralph SA, Speed TP, Baum J. Subcompartmentalisation of Proteins in the Rhoptries Correlates with Ordered Events of Erythrocyte Invasion by the Blood Stage Malaria Parasite. PLoS One. 2012;7(9):e46160.
See original on MMP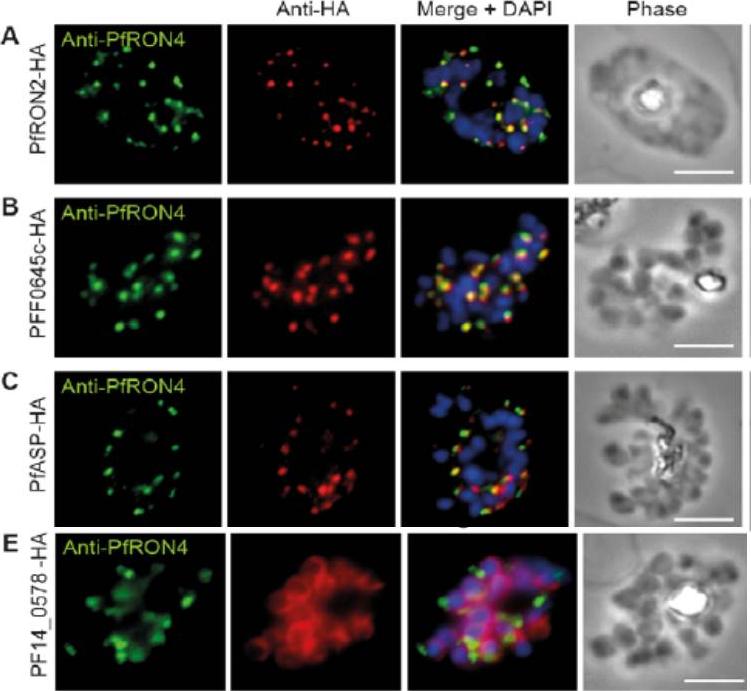
Cellular localisation of invasins in P. falciparum schizonts. (A–E) IFA of schizonts from HA tagged P. falciparum parasite lines labeled with anti-PfRON4, to mark the rhoptry neck, DAPI, to mark nuclei and anti-HA. (A) PfRON2 (B) rhoptry protein PFF0645c (C) apical sushi protein PfASP and (E) inner membrane complex protein PF14_0578. PfRON2-HA schizonts were colabeled with anti-PfRON4, a marker of the rhoptry neck. PFF0645c and PfASP each displayed a similar apical localization in schizonts, showing overlap of labeling with PfRON4. PF14_0578-HA labeling was peripheral in late stage schizonts (E), suggestive of an inner-membrane complex (IMC) or plasma membrane localization.Zuccala ES, Gout AM, Dekiwadia C, Marapana DS, Angrisano F, Turnbull L, Riglar DT, Rogers KL, Whitchurch CB, Ralph SA, Speed TP, Baum J. Subcompartmentalisation of Proteins in the Rhoptries Correlates with Ordered Events of Erythrocyte Invasion by the Blood Stage Malaria Parasite. PLoS One. 2012;7(9):e46160.
See original on MMP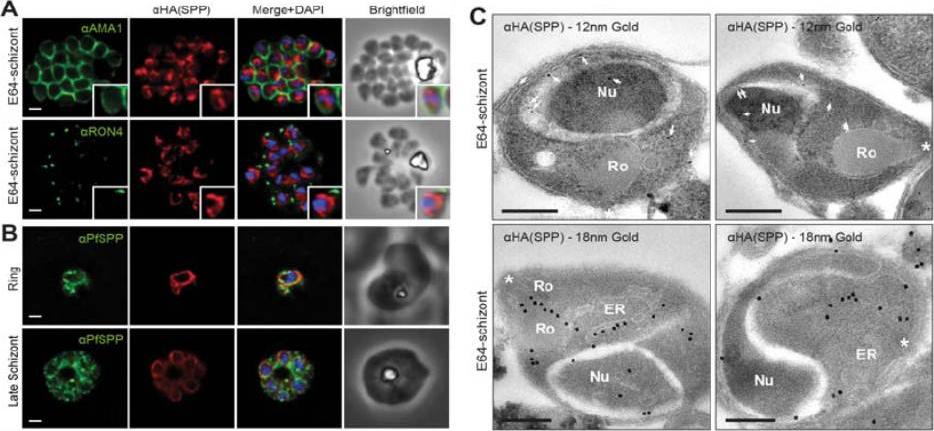
PfSPP localises to the parasite nuclear periphery.(A) IFA of E64 treated PfSPP-HA schizonts labeled with anti-PfAMA1, PfRON4 and HA antibodies. Scale bar 1 μm. Inset displays magnification of single merozoite. (B) IFA of ring and late schizont stage PfSPP-HA parasites labeled with anti-PfSPP and HA antibodies. Scale bar 1 mm. (C) Immuno-electron micrograph of E64 treated PfSPP-HA schizonts with 12 nm or 18 nm immunogold. Arrowheads mark 12 nm immunogold labels and white asterisks marks parasite apex. Nucleus (Nu), endoplasmic reticulum (ER) and rhoptry (Ro). Scale bar = 200 nm. PfSPP is an ER-resident peptidase that remains intracellular throughout the invasion process.Marapana DS, Wilson DW, Zuccala ES, Dekiwadia CD, Beeson JG, Ralph SA, Baum J. Malaria parasite signal peptide peptidase is an ER-resident protease required for growth but not invasion. Traffic. 2012 13(11):1457-65
See original on MMP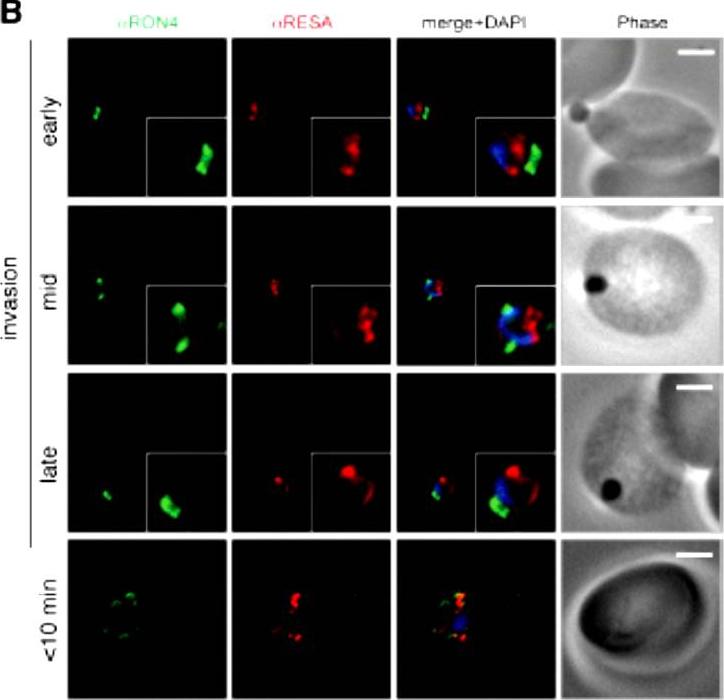
Wide field IFA time course of invasion using anti-RESA/PfRON4. Scale bar = 2.0 μm. Pre-egress shows standard wide-field image. Invasion images show IFA with deconvolution (single slice). RESA is retained within the merozoite body, often at the apical end but frequently basal to rhoptries and micronemes (RESA early). RESA, in contrast, retained its position inside the merozoite, establishing that release of dense granule proteins likely occurs postinvasion (RESA mid). RESA, localization became markedly more peripheral and evenly distributed around the merozoite immediately after invasion (RESA late), supporting the notion that dense granules are released after invasion at the merozoite plasma membrane.Riglar DT, Richard D, Wilson DW, Boyle MJ, Dekiwadia C, Turnbull L, Angrisano F, Marapana DS, Rogers KL, Whitchurch CB, Beeson JG, Cowman AF, Ralph SA, Baum J. Super-resolution dissection of coordinated events during malaria parasite invasion of the human erythrocyte. Cell Host Microbe. 2011 9:9-20.
See original on MMP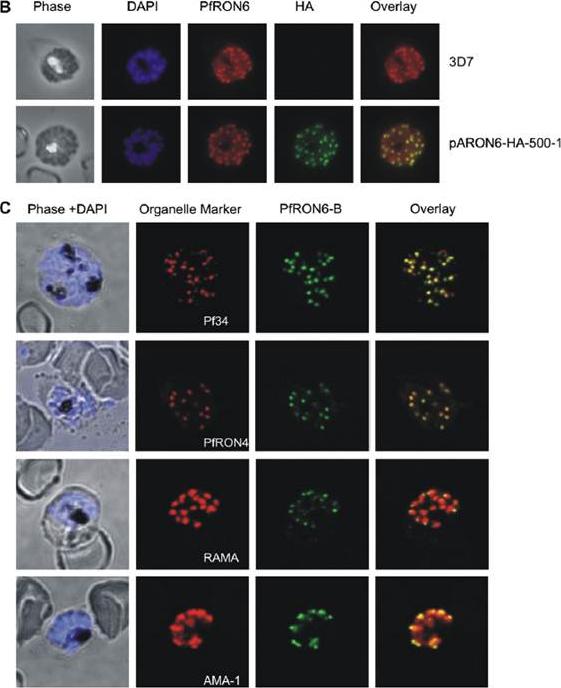
Localisation of the PfRON6 protein in parasitised red blood cells. (B) Double labelling using anti-rPfRON6-B and anti- haemagglutinin (HA) in HA-tagged transgenic parasites. Confocal microscopy using anti-rPfRON6-B with anti-HA. Corresponding overlay images are shown. (C) Double labelling of PfRON6 with Pf34, PfRON4, RAMA or AMA1 in segmented schizonts. Confocal microscopy using anti-rPfRON6-B with anti-Pf34 (rhoptry neck marker), anti-PfRON4 (rhoptry neck marker), anti-RAMA (rhoptry bulb marker), or anti-AMA1 (microneme marker). Corresponding overlay images are shown. a punctate pattern of fluorescence in segmented schizonts characteristic of localisation with the apical secretory organelles.Proellocks NI, Kats LM, Sheffield DA, Hanssen E, Black CG, Waller KL, Coppel RL. Characterisation of PfRON6, a Plasmodium falciparum rhoptry neck protein with a novel cysteine-rich domain. Int J Parasitol. 2008 39(6):683-92. Copyright Elsevier 2009.
See original on MMP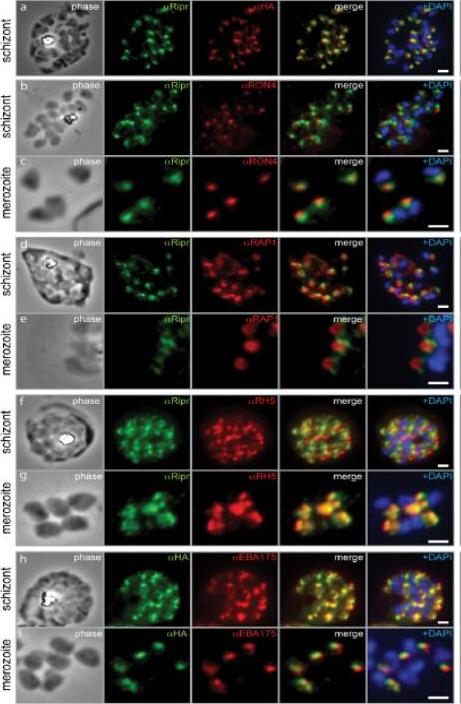
PfRipr localizes to the apical end of merozoites. a) Rabbit polyclonal anti-PfRip antibody (anti-PfRipr/1) recognizes HA-tagged PfRiprHA. b) PfRipr does not co-localize with the rhoptry neck protein RON4 in schizonts. c) PfRipr does not co-localize with the rhoptry neck protein RON4 in merozoites. d) PfRipr does not co-localize with the rhoptry bulb protein, RAP1 in schizonts. e) PfRipr does not co-localize with the rhoptry bulb protein, RAP1 in merozoites. f) PfRipr partially co-localizes with PfRh5 in the schizonts. g) PfRipr mainly co-localizes with PfRh5 in purified merozoites. h) PfRipr co-localizes with the micronemal marker, EBA175, in schizonts. i) PfRipr does not co-localize with the micronemal marker, EBA175, in merozoites.Chen L, Lopaticki S, Riglar DT, Dekiwadia C, Uboldi AD, Tham WH, O'Neill MT, Richard D, Baum J, Ralph SA, Cowman AF. An EGF-like protein forms a complex with PfRh5 and is required for invasion of human erythrocytes by Plasmodium falciparum. PLoS Pathog. 2011 7(9):e1002199.
See original on MMP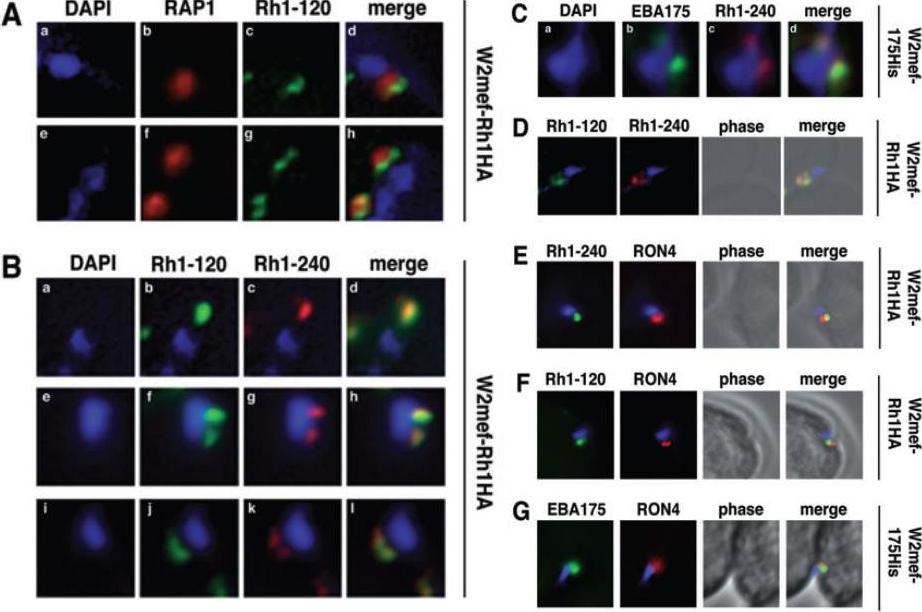
Schizont-processed Rh1-240 colocalizes with Rh1-120 at the apical tip and tight junction of the merozoite. A. The Rh1-120 protein is located at the apical tip and tight junction of merozoites. Free merozoites from the W2mef-Rh1HA line were dual stained with a McAb to RAP1 in the body of the rhoptry and with rat anti-HA antibodies to the tagged 120 kDa Rh1. Anti-HA staining is seen either apical to the RAP1 staining (top panels) or at both sides but posterior to RAP1 staining indicating tight junction staining (bottom panels). Nuclei were stained with DAPI. B. The Rh1-120 protein colocalizes with the Rh1-240 protein at the apical tip and tight junction of merozoites. Free merozoites from the W2mef-Rh1HA line were dual-stained with rat anti-HA antibodies to the tagged 120 kDa Rh1 and with a rabbit antibody (R515) to the 240 kDa Rh1. Colocalization is seen at the apical tip (panels a–d) and at the tight junction (panels e–l) of individual merozoites. W2mef-175His line C. The Rh1-240 protein colocalizes with EBA-175 MAL7P1.176 at the tight junction of merozoites. Free merozoites were dual-stained with mouse anti-His antibodies to tagged EBA-175 and with a rabbit antibody to the 240 kDa Rh1. EBA-175 and Rh1-240 appear to colocalize at the tight junction. D. Free merozoites were dual-stained with rat anti-HA antibodies to the tagged 120 kDa Rh1 and with a rabbit antibody to the 240 kDa Rh1. Simultaneous staining of both the apical tip and the tight junction of a merozoite is seen. E–G. Merozoites were trapped during invasion using cytochalasin D. Rh1-240 was stained with R515, Rh1-120 with rat anti-HA and EBA-175 with mouse anti-His antibodies. PfRON4 was stained either with a mouse antibody (E) or with a rabbit antibody (F and G). Triglia T, Tham WH, Hodder A, Cowman AF. Reticulocyte binding protein homologues are key adhesins during erythrocyte invasion by Plasmodium falciparum. Cell Microbiol. 2009 11:1671-87. Copyright John Wiley & Sons Ltd. 2010.
See original on MMP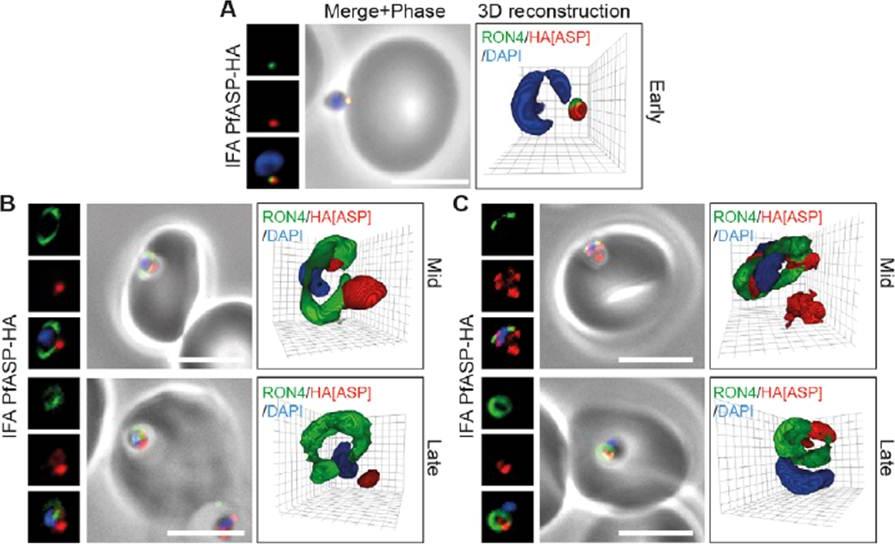
PfASP shows a dual localisation to both the tight junction and merozoite apex during erythrocyte invasion. Widefield 3D imaging of ASP-HA merozoites labeled with anti-HA, anti-PfRON4 and DAPI showing early invasion events (A) and two classes of mid/late invasion (B, C) distributions seen during invasion. Scale bar = 5 mm. 3D reconstruction with 0.2 mm grid intervals. In apically attached parasites, PfASP-HA was located at the apical tip of the merozoite at the same point as PfRON4 (A, Early), consistent with its localisation by IEM to the rhoptry neck in intracellular parasites. Mid invasion, however, when PfRON4 labeling expands to form a ring around invading parasites at the tight junction, PfASPHA was found either predominantly retained at the apical tip of the merozoite or associated with the tight junction (B, C).Zuccala ES, Gout AM, Dekiwadia C, Marapana DS, Angrisano F, Turnbull L, Riglar DT, Rogers KL, Whitchurch CB, Ralph SA, Speed TP, Baum J. Subcompartmentalisation of Proteins in the Rhoptries Correlates with Ordered Events of Erythrocyte Invasion by the Blood Stage Malaria Parasite. PLoS One. 2012;7(9):e46160.
See original on MMP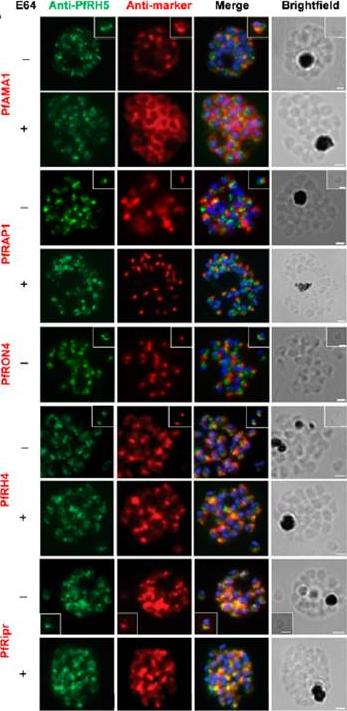
Localization of PfRH5 was assessed by indirect IFA using anti-PfRH5 polyclonal rabbit serum (green). Fixed and permeabilized schizonts with (+) or without (2) E64 treatment or free merozoites (inset) of 3D7 clone P. falciparum were costained with mouse Abs (red) to mark various organelles: PfAMA1 polyclonal (microneme), PfRAP1 mAb (rhoptry body), or PfRON4 mAb (rhoptry neck); or polyclonal mouse serum against further Ags: PfRH4 and PfRipr. Figures show the merge of the dual staining Abs and nuclei stained with DAPI (blue), as well as the brightfield view. Scale bars, 1 mm. anti-PfRH5 rabbit Abs (8) do not colocalize with conventional markers of the rhoptry bulb, rhoptry neck, or micronemes (PfRAP1, PfRON4, and PfAMA1, respectively) in permeabilized schizonts or free merozoites of 3D7 clone P. falciparum parasites. Minimal colocalization in schizonts with PfRH2a/b (data not shown) and also with PfRH4, described as a marker of the rhoptry tip, but significant colocalization with PfRipr in merozoites and late-stage schizonts, especially following treatment with the cysteine protease inhibitor E64, which prevents merozoite release by inhibiting schizont rupture. Impossible to detect PfRH5 on the merozoite surface in any assay, with staining only successful following permeabilization .Douglas AD, Williams AR, Knuepfer E, Illingworth JJ, Furze JM, Crosnier C, Choudhary P, Bustamante LY, Zakutansky SE, Awuah DK, Alanine DG, Theron M, Worth A, Shimkets R, Rayner JC, Holder AA, Wright GJ, Draper SJ. Neutralization of Plasmodium falciparum Merozoites by Antibodies against PfRH5. J Immunol. 2014 192(1):245-58.
See original on MMP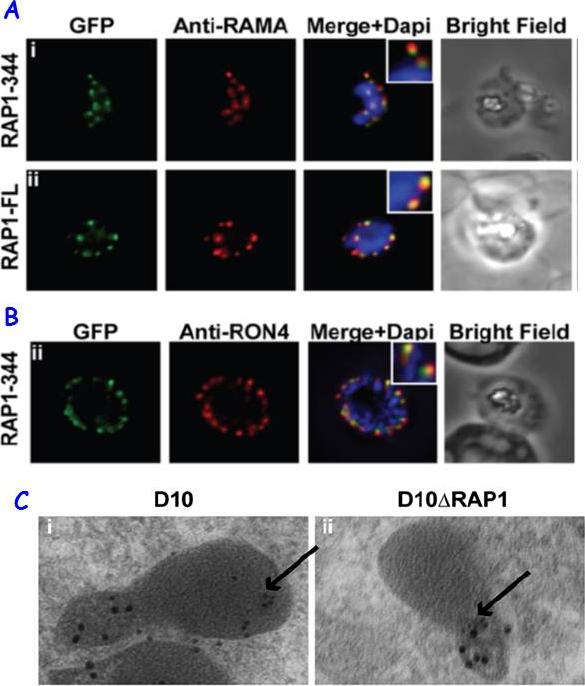
RAP1 contains a bipartite rhoptry signal. A RAP1-344 and RAP1-FL GFP fusions. Both constructs show a punctate fluorescence pattern characteristic of rhoptry localisation. For the RAP1-FL construct, GFP signal overlaps with RAMA. In contrast, the RAP1-344GFP chimera only partially overlaps with RAMA, suggesting rhoptry neck localisation. B. For the RAP1-344 construct, GFP co-localises with the rhoptry neck marker PfRON4. C. Immunoelectron microscopy demonstrates that truncated RAP1 (10 nm beads) in D10DRAP1 parasites is localised in the rhoptry neck, whereas full-length RAP1 in D10 (wild-type) parasites is localised in the rhoptry bulb. PfRON4 PF11_0168 (15 nm beads) is localised in the rhoptry neck in both parasite lines.Richard D, Kats LM, Langer C, Black CG, Mitri K, Boddey JA, Cowman AF, Coppel RL. Identification of Rhoptry Trafficking Determinants and Evidence for a Novel Sorting Mechanism in the Malaria Parasite Plasmodium falciparum. PLoS Pathog. 2009 5(3):e1000328.
See original on MMP
Wide field IFA time course of invasion using A) anti-RAP1/PfRON4. Pre-egress shows standard wide-field image. Invasion images show IFA with deconvolution (single slice). Before invasion commencement, RAP1 clearly remained within the merozoite apex (RAP1 early) posterior to the rhoptry neck as defined by PfRON4. Midinvasion merozoites revealed that RAP1 labeling had moved to an anterior position beginning immediately adjacent to the junction and tracking the exterior of the merozoite as it entered (RAP1 mid). These observations show clearly RAP1 transition pre-, mid-, and postinvasion.Riglar DT, Richard D, Wilson DW, Boyle MJ, Dekiwadia C, Turnbull L, Angrisano F, Marapana DS, Rogers KL, Whitchurch CB, Beeson JG, Cowman AF, Ralph SA, Baum J. Super-resolution dissection of coordinated events during malaria parasite invasion of the human erythrocyte. Cell Host Microbe. 2011 9:9-20.
See original on MMP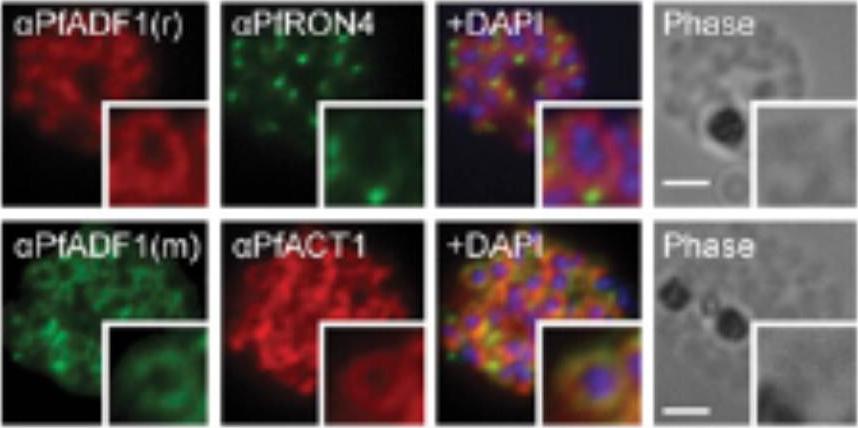
Expression and localization of actin-depolymerizing factor 1 (PfADF1) and actine 1 (PfAct1) in P. falciparum. IFA of mature P. falciparum schizont stages labeled with PfAct1, PfADF1, and PfRON4 antisera. r, rabbit; m, mouse. (Scale bars, 2 μm.) Immunofluorescence assays (IFA) of schizont stages (before merozoite release) demonstrated broad labeling across the parasite cytosol with the exception of the nucleus (marked by DAPI. This distribution was consistent with that of PfAct1.Wong W, Skau CT, Marapana DS, Hanssen E, Taylor NL, Riglar DT, Zuccala ES, Angrisano F, Lewis H, Catimel B, Clarke OB, Kershaw NJ, Perugini MA, Kovar DR, Gulbis JM, Baum J. Minimal requirements for actin filament disassembly revealed by structural analysis of malaria parasite actin-depolymerizing factor 1. Proc Natl Acad Sci U S A. 2011 108:9869-74.
See original on MMP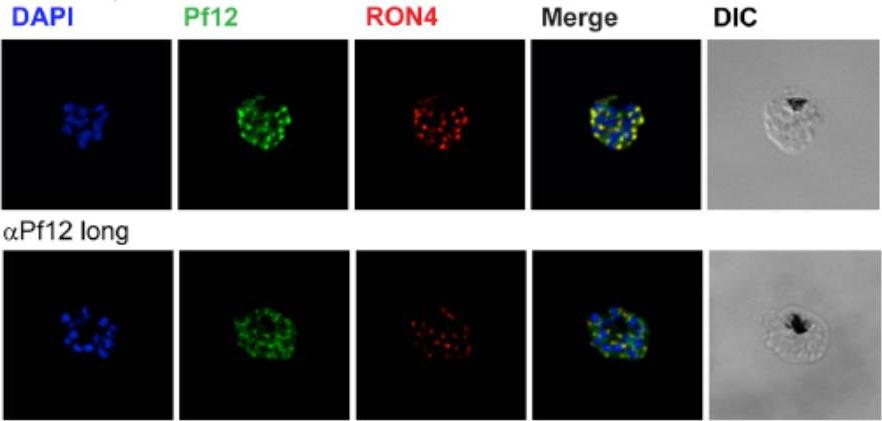
Localization of Pf12 shows punctate apical staining with anti-Pf12D2 antibody and more diffuse staining with anti-Pf12long antibody. Localization of Pf12 on mature schizonts. Confocal microscopy images of purified late-schizont infected RBCs are shown. Top panel shows labeling with affinity purified anti-Pf12D2 (against C-terminal domain), and anti-RON4 antibodies. Bottom panel shows labeling with affinity purified anti-Pf12long (against full length), and anti-RON4 antibodies. Simultaneous labeling of PfRON4, a rhoptry neck protein, consistently shows adjacent localization to Pf12 with a significant degree of co-localization. These data indicate that in the late schizont stages, Pf12 is found in an apical organelle.Tonkin ML, Arredondo SA, Loveless BC, Serpa JJ, Makepeace KA, Sundar N, Petrotchenko EV, Miller LH, Grigg ME, Boulanger MJ. Structural and biochemical characterization of Plasmodium falciparum 12 (Pf12) reveals a unique inter-domain organization and the potential for an antiparallel arrangement with Pf41. J Biol Chem. 2013 288(18):12805-17
See original on MMP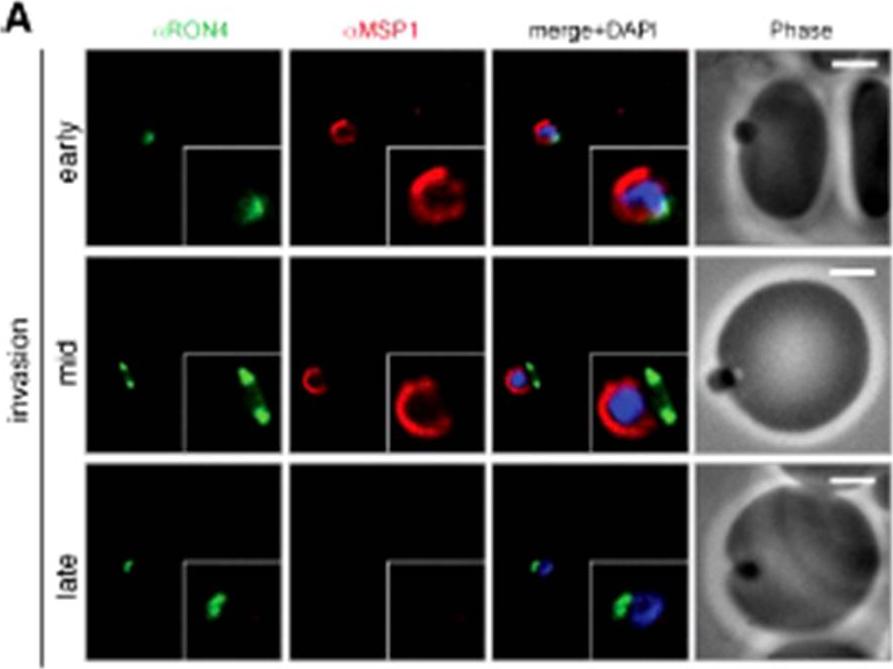
Wide field IFA time course of invasion using A) anti-MSP1/PfRON4. Scale bar = 2.0 μm. Before commencement of invasion, no loss of MSP1 labeling was apparent (MSP1 early). Loss of MSP1 tracked the exterior of invading merozoites concurrent with different stages of passage through the tight (MSP1 mid). The lack of contiguity between shed MSP1 and PfSUB2 sheddase, each in comparison with PfRON4 suggests that MSP1 may be processed by PfSUB2 early in invasion but shed only during passage through the junction.Riglar DT, Richard D, Wilson DW, Boyle MJ, Dekiwadia C, Turnbull L, Angrisano F, Marapana DS, Rogers KL, Whitchurch CB, Beeson JG, Cowman AF, Ralph SA, Baum J. Super-resolution dissection of coordinated events during malaria parasite invasion of the human erythrocyte. Cell Host Microbe. 2011 9:9-20.
See original on MMP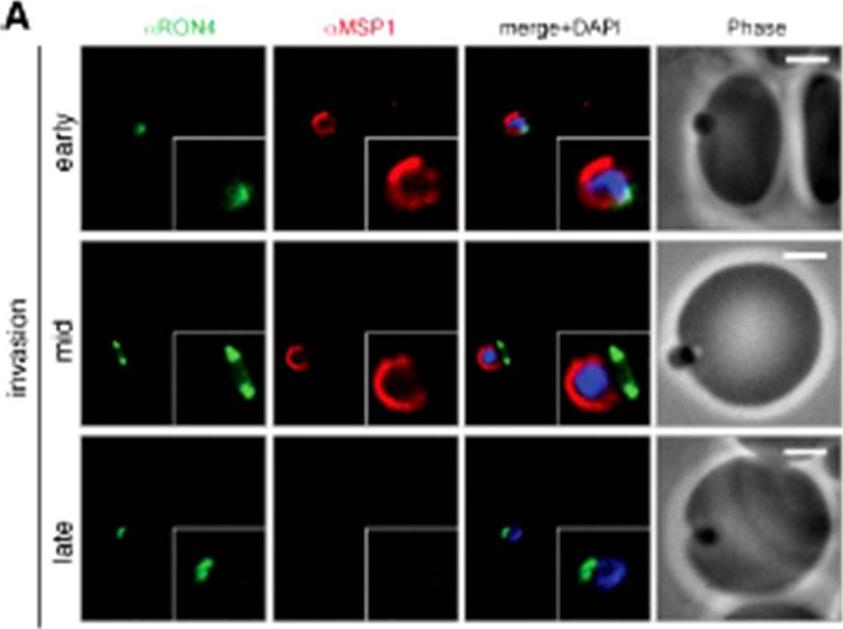
Wide field IFA time course of invasion using A) anti-MSP1/PfRON4. Scale bar = 2.0 μm. Before commencement of invasion, no loss of MSP1 labeling was apparent (MSP1 early). Loss of MSP1 tracked the exterior of invading merozoites concurrent with different stages of passage through the tight (MSP1 mid). The lack of contiguity between shed MSP1 and PfSUB2 sheddase, each in comparison with PfRON4 suggests that MSP1 may be processed by PfSUB2 early in invasion but shed only during passage through the junction.Riglar DT, Richard D, Wilson DW, Boyle MJ, Dekiwadia C, Turnbull L, Angrisano F, Marapana DS, Rogers KL, Whitchurch CB, Beeson JG, Cowman AF, Ralph SA, Baum J. Super-resolution dissection of coordinated events during malaria parasite invasion of the human erythrocyte. Cell Host Microbe. 2011 9:9-20.
See original on MMP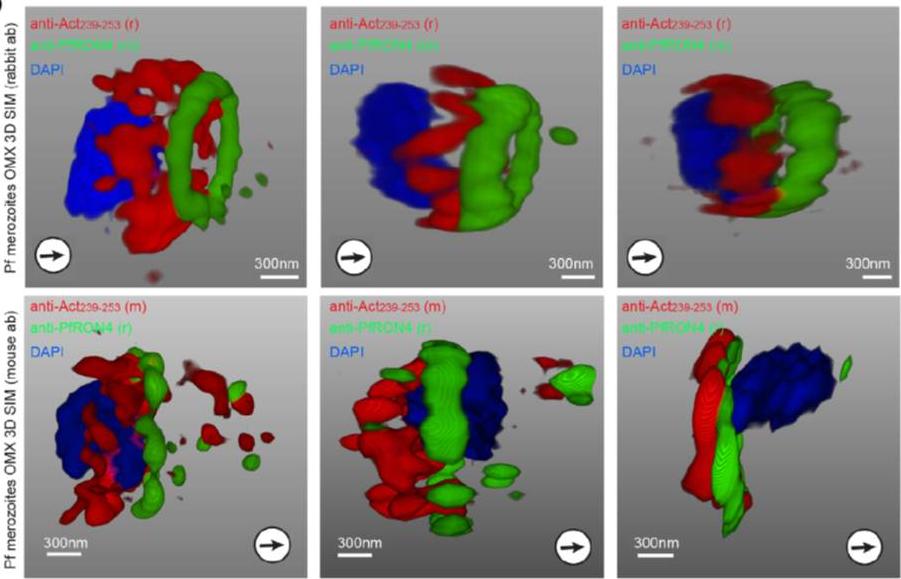
3D structured illumination microscopy (3D SIM) of three separate invading P. falciparum merozoites labelled with rabbit (upper row) and mouse (lower row) anti-Act239–253. Labelling shows actin (Red), RON4 (Green) and DAPI (Blue). In all instances Anti-Act239–253 labelling was concentrated in a ring lying posterior to the tight junction during merozoite invasion, defined as the edge of the junction towards the posterior of the parasite.Angrisano F, Riglar DT, Sturm A, Volz JC, Delves MJ, Zuccala ES, Turnbull L, Dekiwadia C, Olshina MA, Marapana DS, Wong W, Mollard V, Bradin CH, Tonkin CJ, Gunning PW, Ralph SA, Whitchurch CB, Sinden RE, Cowman AF, McFadden GI, Baum J. Spatial Localisation of Actin Filaments across Developmental Stages of the Malaria Parasite. PLoS One. 2012;7(2):e32188.
See original on MMP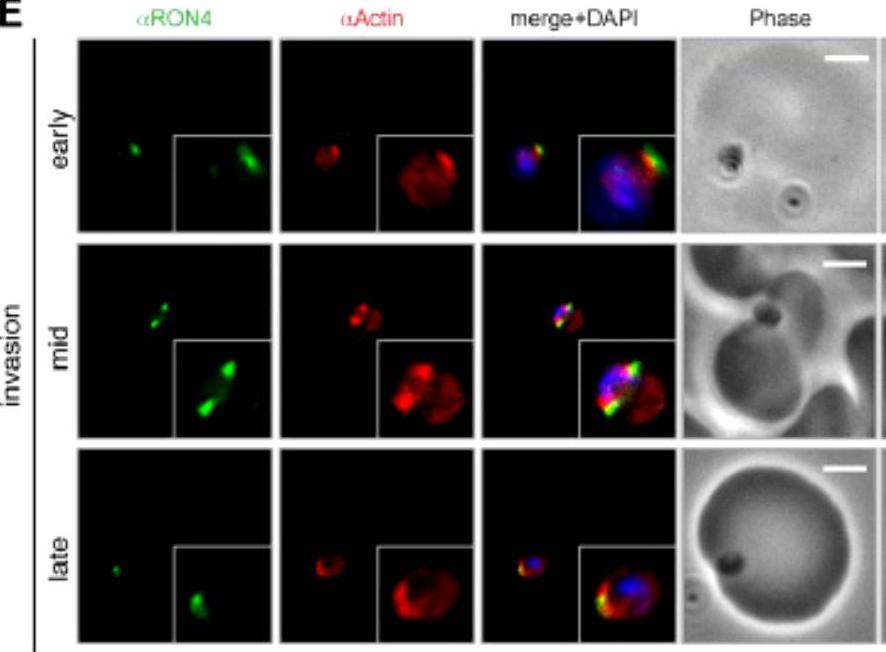
Wide field IFA time course of invasion using anti-PfActin/PfRON4. IFA scale bar = 2.0 μm. The distribution of actin within the merozoite was concentrated at the parasite apex, with diffuse labeling elsewhere in the cytoplasm (Actin early). Actin fluorescence could be detected, concentrated at the tight junction (Actin mid). In the latter stages of invasion, the concentration of actin labeling was at the rear of the invaded merozoite (Actin late).Riglar DT, Richard D, Wilson DW, Boyle MJ, Dekiwadia C, Turnbull L, Angrisano F, Marapana DS, Rogers KL, Whitchurch CB, Beeson JG, Cowman AF, Ralph SA, Baum J. Super-resolution dissection of coordinated events during malaria parasite invasion of the human erythrocyte. Cell Host Microbe. 2011 9:9-20.
See original on MMP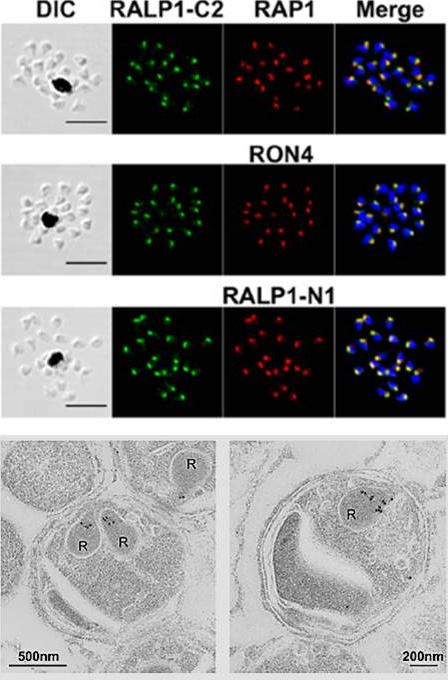
Localization of RALP1 in P. falciparum merozoites. RALP1 localization shown by an immunofluorescence assay. Paraformaldehyde-fixed mature schizonts were probed with rabbit anti-RALP1-C2 (green; C-terminal region of RALP1, encompassing 315 aa [N396 to K710]) and mouse anti-RAP1 (rhoptry bulb marker) antibodies (top) or anti-RON4 (rhoptry neck marker) (middle) or anti-RALP1-N1 (bottom; red; C-terminal region of RALP1, encompassing 239 aa [N396 to P634]) antibody. Parasite nuclei were stained with DAPI (blue). Scale bars represent 5 mm. DIC, differential interference contrast. (E) RALP1 localization shown by IEM. Two sections of merozoites in schizont-infected erythrocytes probed with purified rabbit anti-RALP1-C2 antibody and subsequently with a secondary antibody conjugated with gold particles are shown. The black dots indicate signals from gold particles localized in rhoptry necks. R, rhoptry.Ito D, Hasegawa T, Miura K, Yamasaki T, Arumugam TU, Thongkukiatkul A, Takeo S, Takashima E, Sattabongkot J, Han ET, Long CA, Torii M, Tsuboi T. RALP1 is a rhoptry neck erythrocyte-binding protein of Plasmodium falciparum merozoites and a potential blood-stage vaccine candidate antigen. Infect Immun. 2013 81(11):4290-8.
See original on MMP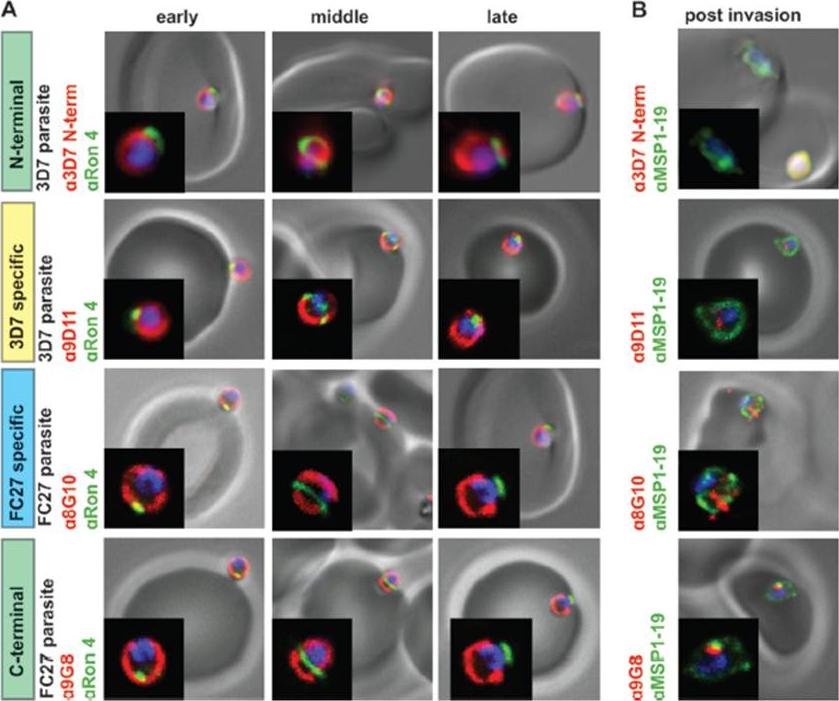
GPI-anchored MSP2 is carried into invaded RBCs and rapidly degraded. (A) Invading merozoites were labeled with the tight junction marker PfRON4(green) and colabeled with antibodies to different regions of MSP2 (red). All regions of MSP2 were carried through the tight junction into the RBC, with labeling being visible on both sides of PfRON4 at the tight junction. (B) Newly invaded rings (10 min postinvasion) were labeled with antibodies to MSP1-19 (green) and antibodies to different regions of MSP2 (red). MSP2 was rapidly degraded postinvasion, with little or no labeling being visible in rings. Images are single slices from deconvoluted stacks. Representative images of antibodies to the different regions of MSP2; other antibodies tested showed the same labeling patterns. The MSP2 allele of the parasite strain used in each row of images is indicated as 3D7 parasite or FC27 parasite (D10 parasite strain). Antibody dilutions used were 1:500 for secondary antibodies, 1:250 for 8G10, 1:100 for PfRON4, and 1:50 for N-terminal purified antibodies, 9D11, and9G8.Boyle MJ, Langer C, Chan JA, Hodder AN, Coppel RL, Anders RF, Beeson JG. Sequential processing of merozoite surface proteins during and after erythrocyte invasion by Plasmodium falciparum. Infect Immun. 2014 82(3):924-36.
See original on MMP
MSP4 is carried into RBCs during invasion without cleavage. (A) Invading 3D7 merozoites were labeled with PfRON4 (green) and antibodies raised against full-length MSP4 (red). MSP4 labeling was visible on both sides of the tight junction for early-, mid-, and late-stage invading parasites. (B) D10 strain merozoites were labeled with PfRON4 (green) and antibodies raised against different regions of MSP4 (red). All MSP4 antibodies labeled merozoites on both sides of the tight junction. Images are representative of mid- and late-stage invading parasites. N-terminal (anti-MSP4A) and C-terminal (anti-MSP4D) regions of MSP4 were carried into RBCs during invasion, without any detectable cleavage and shedding (C) MSP4 is present at 5 h postinvasion. Isolated 3D7 strain merozoites were allowed to invade and fixed at 10 min and 2 and 5 h postinvasion. Ring-stage parasites were labeled with MSP1-19 antibodies (red) and full-length MSP4 (green). Positive MSP4 labeling was seen at 5 h postinvasion. Antibody concentrations used were 1:500 for secondary antibodies and 1:50 for all MSP4 antibodies.Boyle MJ, Langer C, Chan JA, Hodder AN, Coppel RL, Anders RF, Beeson JG. Sequential processing of merozoite surface proteins during and after erythrocyte invasion by Plasmodium falciparum. Infect Immun. 2014 82(3):924-36.
See original on MMP
PfMyoB-GFP is located at the apical pole of the merozoite. Indirect immunofluorescence of PfMyoB-GFP (green) with various merozoite organelle markers (red), using antisera to i) EBA175 (microneme marker), ii) RAP1 (rhoptry bulb), iii) RON4 (rhoptry neck) and iv) α-tubulin. Samples were counterstained with DAPI (blue). The merged images are also shown. Rows i-iii show individual schizonts, row iv shows an individual merozoite. Scale bar: 2 μM. Antibodies to GFP produced a compact discrete dot pattern of fluorescence located at the very apical end of the MyoB-GFP parasites, near to the localisation of the apical markers EBA175, RON4, and RAP1. However, whilst in some cases the fluorescent signal partially overlapped with these markers, MyoB appeared to be in a distinct location within the cell, anterior to the microneme marker, the rhoptry bulb, and even to the rhoptry neck.Yusuf NA, Green JL, Wall RJ, Knuepfer E, Moon RW, Schulte-Huxel C, Stanway RR, Martin SR, Howell SA, Douse CH, Cota E, Tate EW, Tewari R, Holder AA. The Plasmodium Class XIV Myosin, MyoB has a Distinct Subcellular Location in Invasive and Motile Stages of the Malaria Parasite, and an Unusual Light Chain. J Biol Chem. 2015 Mar 23.
See original on MMP
PfMyoB-GFP remains at the anterior of the merozoite during invasion of the host cell.MyoB-GFP-expressing P. falciparum parasites were fixed during various stages of invasion, then MyoBGFP (green) was revealed using rabbit anti-GFP antibodies, RON4 (red) was detected using mAb 24C6 and nuclei stained with DAPI (blue). Merged images including the DIC are shown, as well as a cartoon of the invasion stage in which the moving junction is shown by the blue arrowheads, the extracellular merozoite is grey, and the intracellular parasite is denoted by a dotted line and is uncoloured. The invasion steps have been divided into initial attachment, followed by early and late stages of invasion as well as the final steps of invasion with the release of the remnant junction and formation of the ring stage. Scale bar: 2 μM.Yusuf NA, Green JL, Wall RJ, Knuepfer E, Moon RW, Schulte-Huxel C, Stanway RR, Martin SR, Howell SA, Douse CH, Cota E, Tate EW, Tewari R, Holder AA. The Plasmodium Class XIV Myosin, MyoB has a Distinct Subcellular Location in Invasive and Motile Stages of the Malaria Parasite, and an Unusual Light Chain. J Biol Chem. 2015 Mar 23.
See original on MMP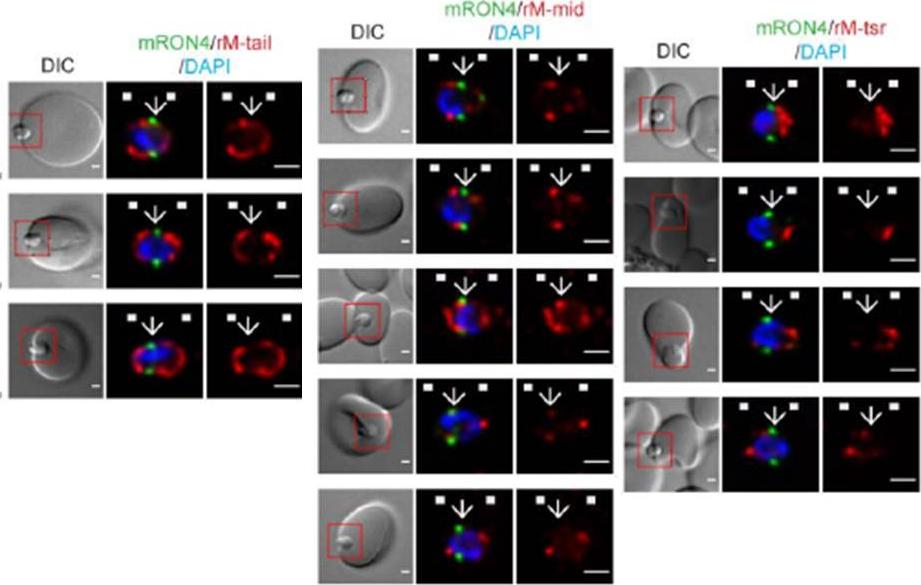
Longitudinal intensity profiling identifies unexpected localisation profiles for MTRAP during merozoite invasion. A-C). Single-slice images of example merozoites of mRON4 (green) vs (Left panle) rMTRAP-tail (rM-tail) (red), (midle panel) rMTRAP mid (rM-mid) (red), or (right panel) rMTRAP tsr (rM-tsr). Scale bars = 1 μm. Red boxes in DIC images indicate zoomed regions for middle and right panels. Arrows indicate the position of the RON4 labelled tight junction.Riglar DT, Whitehead L, Cowman AF, Rogers KL, Baum J. Localization-based imaging of malarial antigens during red cell entry reaffirms role for AMA1 but not MTRAP in invasion. J Cell Sci. 2015. [Epub ahead of print]
See original on MMP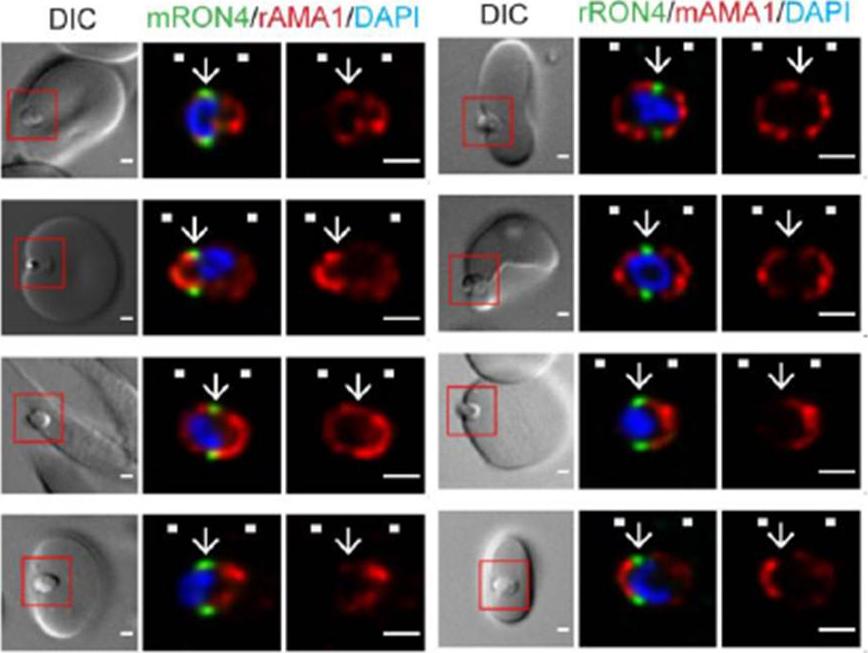
Longitudinal intensity profiling demonstrates antibody specific variation in AMA1 localisation at the tight junction. Single-slice images of example merozoites (left panel) mRON4 (green) vs rAMA1 (red) or(right panel) rRON4 (green) vs mAMA1 (red). Scale bars = 1 μm. Red boxes in DIC images indicate zoomed regions for middle and right panels. Arrows indicate the position of the RON4 labelled tight junctionRiglar DT, Whitehead L, Cowman AF, Rogers KL, Baum J. Localization-based imaging of malarial antigens during red cell entry reaffirms role for AMA1 but not MTRAP in invasion. J Cell Sci. 2015. [Epub ahead of print]
See original on MMP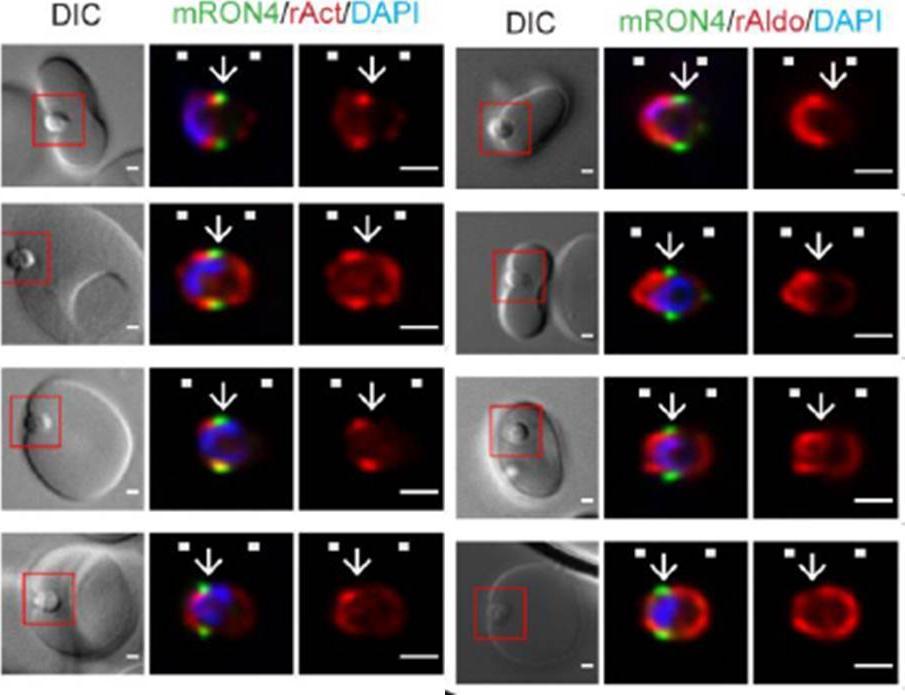
Longitudinal intensity profiling localises actin and aldolase towards the rear of the invading merozoite’s tight junction. Single-slice images of example merozoites for the longitudinal intensity profiling of mRON4 (green) vs (left panel) rActin (rAct) (red) or (right panel) rAlodlase (rAldo) (red). Scale bars = 1 μm. Red boxes in DIC images indicate zoomed regions for middle and right panels. Arrows indicate the position of the RON4 labelled tight junctionRiglar DT, Whitehead L, Cowman AF, Rogers KL, Baum J. Localization-based imaging of malarial antigens during red cell entry reaffirms role for AMA1 but not MTRAP in invasion. J Cell Sci. 2015. [Epub ahead of print] PMID:
See original on MMP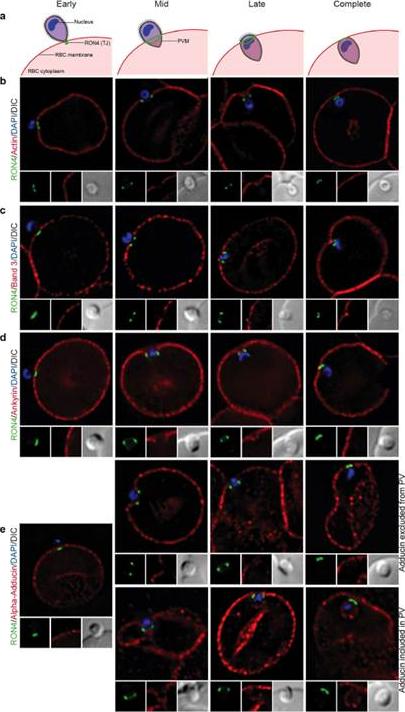
Imaging of the erythrocyte cytoskeleton throughout merozoite invasion. Widefield deconvolution fluorescence imaging of fixed merozoite invasion samples labelled with anti-RON4, DAPI and markers of erythrocyte cytoskeletal proteins. (a) Schematic showing how RON4 labelling allows discernment of early, mid, late and complete invasion events. Early in invasion, during apical attachment, RON4 appears as a single point of fluorescence before expanding to form a ring around the invading merozoite as invasion progresses. When imaged in two dimensions, this ring appears as two single points of fluorescence on either side of the merozoite. The right junction marks the boundary between the erythrocyte plasma membrane and the parasitophorous vacuole membrane, and closes at the base of the parasite at the end of invasion. (b) Erythrocyte actin, labelled by phalloidin-Alexa594. (c,d) Band 3, ankyrin and adducin in invasion labelled with specific antibodies. PVM, parasitophorous vacuole membrane; RBC, red blood cell; TJ, tight junctionZuccala ES, Satchwell TJ, Angrisano F, Tan YH, Wilson MC, Heesom KJ, Baum J.Quantitative phospho-proteomics reveals the Plasmodium merozoite triggerspre-invasion host kinase modification of the red cell cytoskeleton. Sci Rep. 2016Feb 2;6:19766. doi: 10.1038/srep19766.
See original on MMP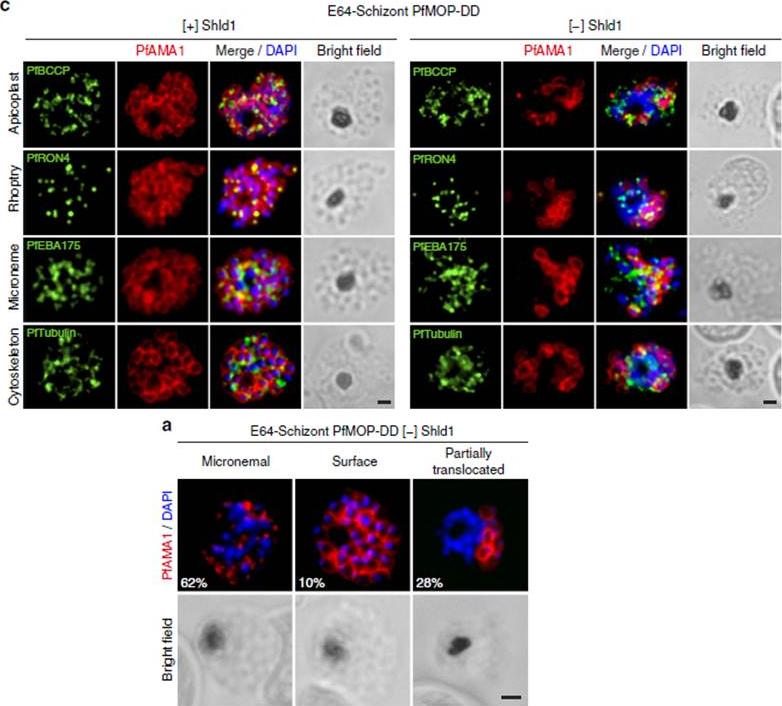
PfAMA1 translocation is aberrant in schizonts with PfMOP-knockdown. (a) Schizonts from [+]/[-] Shld1 PfMOP-DD parasites were E64-treated, fixed, probed with anti-PfAMA1 and scored as micronemal (M), partially translocated (PT) or surface (S), representative [+] Shld1 parasite IFAs shown. (c) Synchronized schizont stage (40–44 h) parasites, maintained with 250nM (left panel) or 0 nM (right panel) Shld1, were incubated 6 h in presence of 10 mM E64, methanol-fixed, permeabilized, and stained using antibodies against PfRON4, PfRhopH3, PfEBA175 and PfTubulin. Staining for these markers was similar in [+] and [-] Shld1 conditions. PfAMA1 staining was used to identify E64-treated schizonts that were sufficiently mature (that is, surface staining or partially translocated staining, but not micronemal staining, scale bar, 1 mm).Absalon S, Robbins JA, Dvorin JD. An essential malaria protein defines the architecture of blood-stage and transmission-stage parasites. Nat Commun. 2016 7:11449.
See original on MMP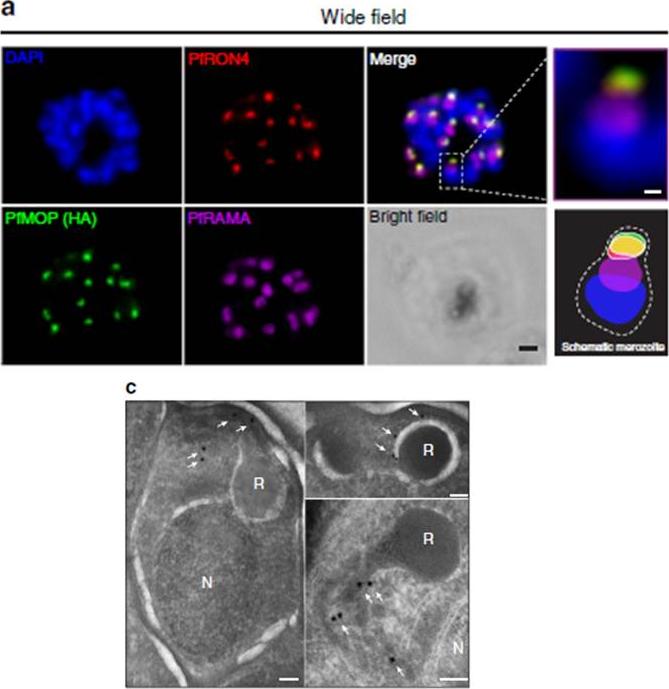
PfMOP localizes to apical area of parasites. Representative pictures of E64-treated schizont-stages. (a) Wide-field IFA of segmented schizont stained with antibodies against PfRAMA, PfRON4, and HA. Scale bar, 1 mm (c) Transmission electron microscopy with (white arrows) gold-labelled antibodies against HA (N, nucleus; R, rhoptry, scale bar, 100 nm).Absalon S, Robbins JA, Dvorin JD. An essential malaria protein defines the architecture of blood-stage and transmission-stage parasites. Nat Commun. 2016 7:11449.
See original on MMP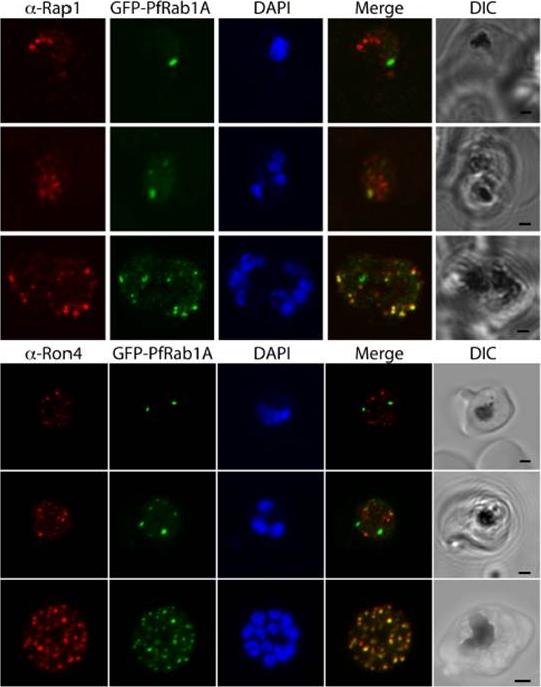
GFP-PfRab1A fluorescence in late schizonts is associated with rhoptry markers. Rhoptry markers Rap1 and Ron4 are found in trophozoites and schizonts as discrete foci that colocalize with GFP-PfRab1A fluorescence in late schizonts, but not in earlier phases of parasite development.Morse D, Webster W, Kalanon M, Langsley G, McFadden GI. Plasmodium falciparum Rab1A Localizes to Rhoptries in Schizonts. PLoS One. 2016 Jun 27;11(6):e0158174
See original on MMP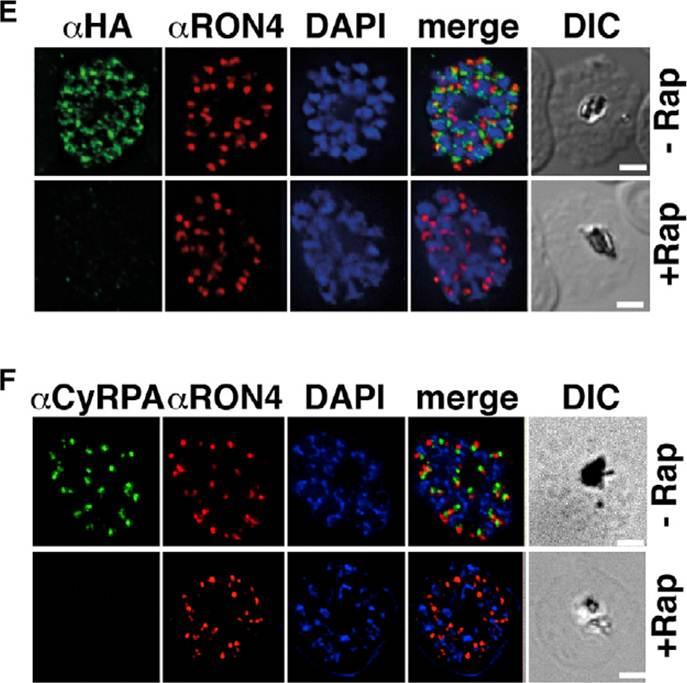
Previous attempts to disrupt PfRipr and CyRPA in P. falciparum have not been successful, suggesting their function is essential. To understand their function, we constructed P. falciparum lines in which pfripr and cyrpa could be conditionally deleted using dimerizable Cre recombinase (DiCre) by generating RiprloxCre and CyRPAloxCre. The absence of PfRipr and CyRPA expression was analyzed by immunofluorescence, and a significant proportion of the populations showed no detectable expression (E and F). These results show that rapamycin-treated RiprloxCre and CyRPAloxCre have substantially decreased expression of PfRipr and CyRPA, respectively. The decrease in expression had no effect on expression and localization of other proteins involved in merozoite invasion.Volz JC, Yap A, Sisquella X, Thompson JK, Lim NT, Whitehead LW, Chen L, Lampe M, Tham WH, Wilson D, Nebl T, Marapana D, Triglia T, Wong W, Rogers KL, Cowman AF. Essential Role of the PfRh5/PfRipr/CyRPA Complex during Plasmodium falciparum Invasion of Erythrocytes. Cell Host Microbe. 2016 Jun 30 [Epub ahead of print]
See original on MMP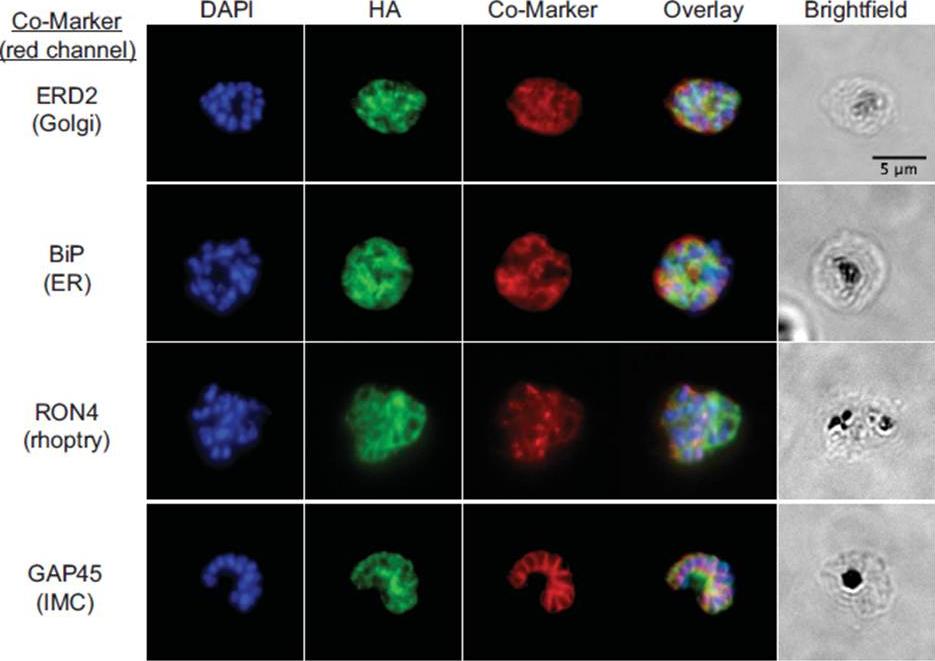
fixed schizont stage parasites were stained with rat α-HA antibody and co-stained with rabbit anti-PfERD2 (1:200), rabbit anti-PfBiP (1:500), mouse anti-PfRON4 (1:100), or rabbit anti-PfGAP45 (1:1000). Primary antibodies were detected with Alexa488-conjugated goat α-rat antibody (1:1000) and Alexa555-conjugated goat α-rabbit or goat anti-mouse (1:1000).Blomqvist K, DiPetrillo C, Streva VA, Pine S, Dvorin JD. Receptor for Activated C-Kinase 1 (PfRACK1) is required for Plasmodium falciparum intra-erythrocytic proliferation. Mol Biochem Parasitol. 2016 Oct 9. pii: S0166-6851(16)30129-3.
See original on MMP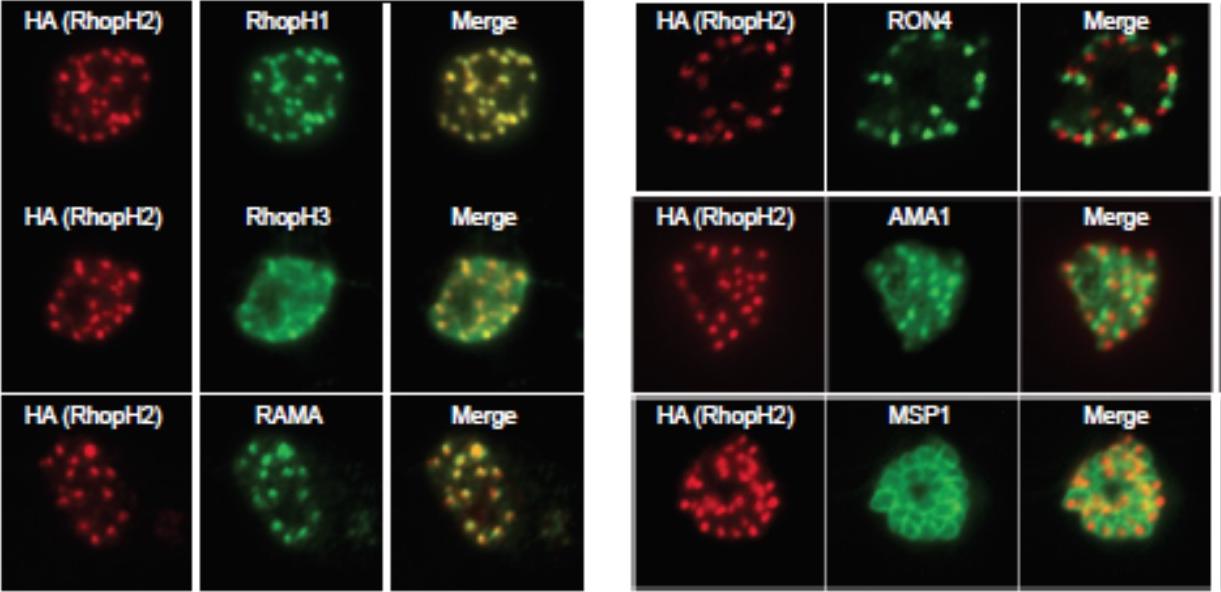
Generation of transgenic parasites in which RhopH2 is epitope-tagged. Immunofluorescence analysis (IFA) on schizonts fixed with acetone/methanol and labelled with anti-HA antibody to detect RhopH2 and other antibodies, as indicated. Immunofluorescence analysis (IFA) confirmed RhopH2-HA localized to the rhoptry and co-localized with other rhoptry bulb proteins, RhopH1, RhopH3 and RAMA but not with the rhoptry neck protein, RON4, the micronemal marker, AMA-1 or the plasma membrane protein MSP1.Counihan N, Chisholm SA, Bullen HE, Srivastava A, Sanders PR, Jonsdottir TK, Weiss GE, Ghosh S, Crabb BS, Creek DJ, Gilson PR, de Koning-Ward TF. Plasmodium falciparum parasites deploy RhopH2 into the host erythrocyte to obtain nutrients, grow and replicate. Elife. 2017 Mar 2;6. pii: e23217
See original on MMP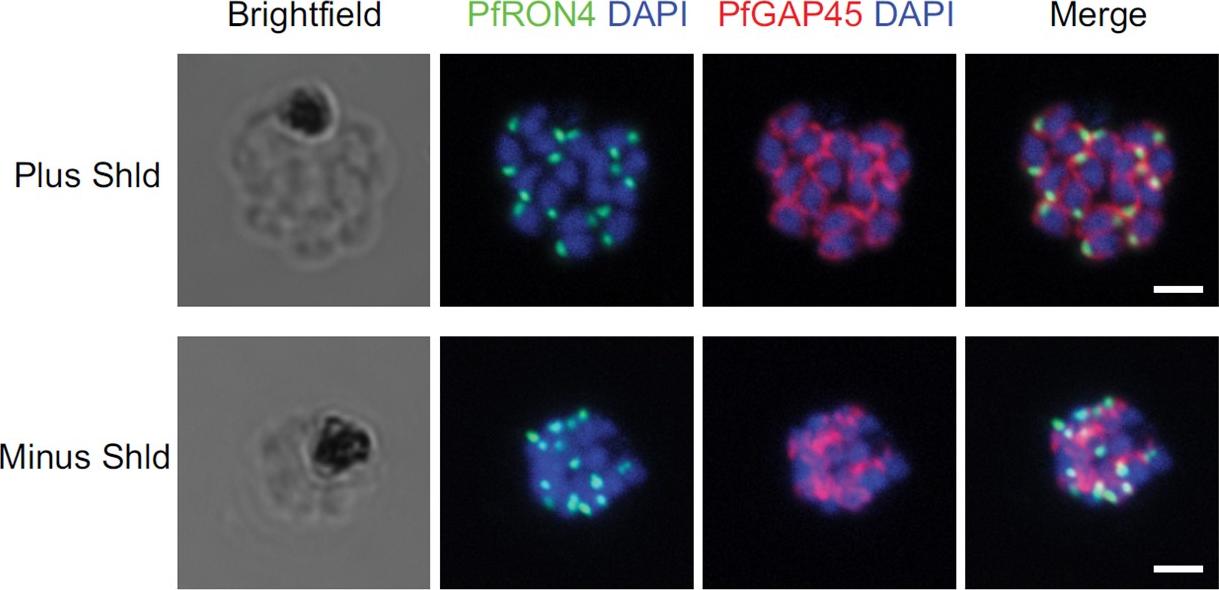
IFA of parasites grown in the presence and absence of Shld probed with antibodies against PfRON4 (green) and PfGAP45 (red). Bars, 2 mm. Staining for PfGAP45, a soluble protein that complexes with PfGAP50 (along with PfMyoA and PfMTIP) to localize to the inner membrane complex (IMC), revealed readily detected expression of the PfGAP45 protein but the absence of normal morphology in PfCyc1-deficient parasites. In contrast, IFAs against the rhoptry protein PfRON4 suggests unencumbered rhoptry formation. Similarly, IFA against the In contrast, IFAs against the rhoptry protein PfRON4 suggests unencumbered rhoptry formation.Robbins JA, Absalon S, Streva VA, Dvorin JD. The Malaria Parasite Cyclin H Homolog PfCyc1 Is Required for Efficient Cytokinesis in Blood-Stage Plasmodium falciparum. MBio. 2017 Jun 13;8(3). pii: e00605-17.
See original on MMP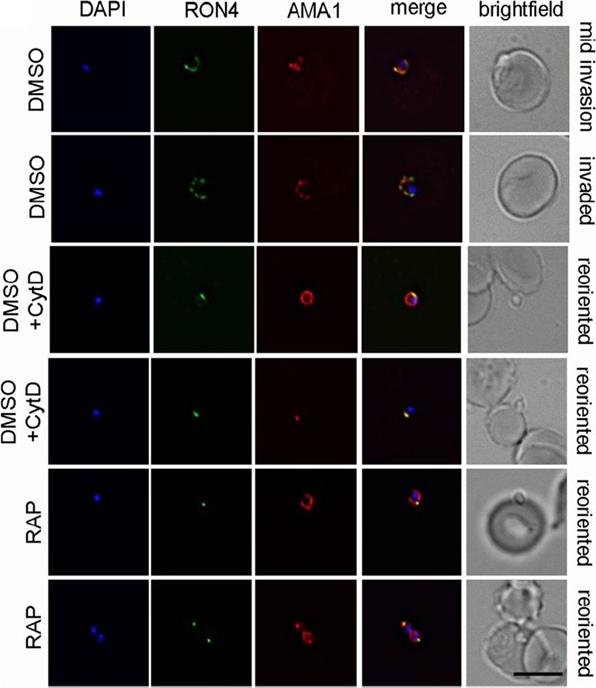
PfACT1 KO merozoites can secrete their micronemes and form a tight junction (TJ) but cannot invade erythrocytes. IFA of TJ formation. Colocalisationof rhoptry neck protein 4 (RON4) and AMA1 at the merozoite-erythrocyte boundary indicates successful TJ formation in DMSO controls (upper twopanels), controls treated with cytochalasin D (middle two panels) and in PfACT1 KOs (lower two panels). Seventy-six percent of DMSO control parasitesinvaded erythrocytes in the time frame of the assa. In contrast, 84% of RAP-treated parasites attached to the erythrocyte and could undergo reorientationand appeared to secrete RON proteins which are required for formation of the junction. However, a typical circular junction could never be observed andparasites were incapable of invading erythrocytes demonstrating a critical requirement for parasite actin for host cell invasion.Das S, Lemgruber L, Tay CL, Baum J, Meissner M. Multiple essential functions of Plasmodium falciparum actin-1 during malaria blood-stage development. BMC Biol. 2017 Aug 15;15(1):70.
See original on MMP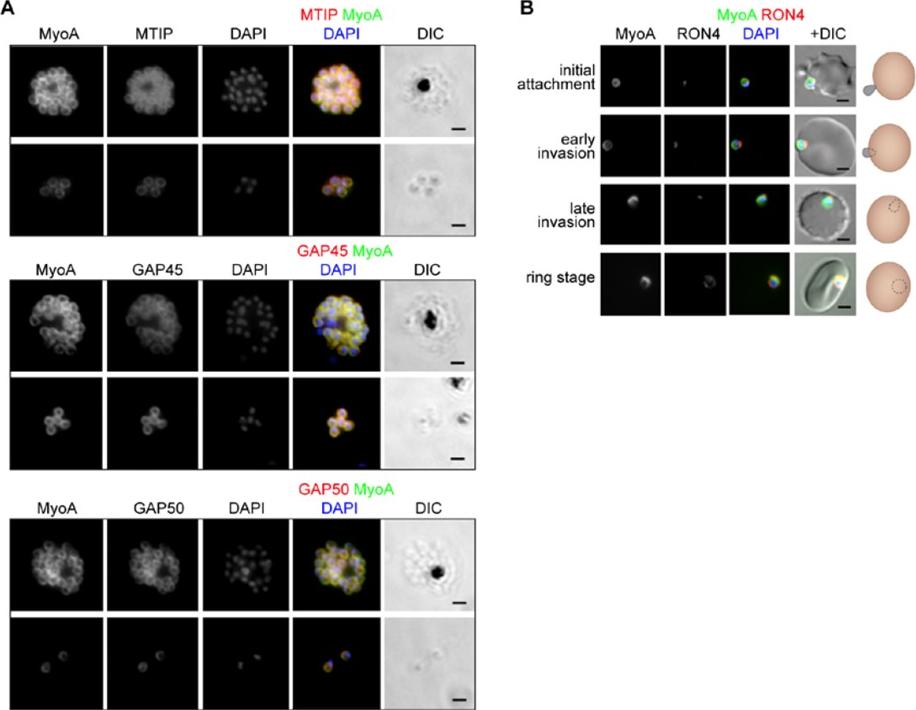
The location of MyoA during P. falciparum merozoite invasion. (A) MyoA-GFP is located at the periphery of developing intracellular (top row of each pair of images) and free extracellular merozoites (bottom row), and is colocalized with antibodies specific for IMC proteins MTIP, GAP45 and GAP50. In the merged colour image the MyoA-GFP signal is green and antibodies specific for the IMC proteins are red; nuclei are stained blue with DAPI. The DIC image is also shown. (B) Individual merozoites are captured at different stages of invasion from initial attachment, through early and late invasion to the intracellular ring stage. MyoA-GFP remains peripheral whereas RON4, initially in the apical rhoptry neck, relocates during invasion. Merged colour images with MyoA-GFP (green), RON4 (red), and nuclei (blue) and DIC images are also shown, together with a schematic of each cell-pair. Scale bar: 2 μm.Green JL, Wall RJ, Vahokoski J, Yusuf NA, Ridzuan MAM, Stanway RR, Stock J, Knuepfer E, Brady D, Martin SR, Howell SA, Pires IP, Moon RW, Molloy JE, Kursula I, Tewari R, Holder AA. Compositional and expression analyses of the glideosome during the Plasmodium life cycle reveal an additional myosin light chain required for maximum motility. J Biol Chem. 2017 Sep 11.
See original on MMPMore information
| PlasmoDB | PF3D7_1116000 |
| GeneDB | PF3D7_1116000 |
| Malaria Metabolic Pathways | Localisation images Pathways mapped to |
| Previous ID(s) | PF11_0168 |
| Orthologs | PBANKA_0932000 , PCHAS_0912300 , PKNH_0913700 , PVP01_0916600 , PVX_091434 , PY17X_0934000 |
| Google Scholar | Search for all mentions of this gene |