PF3D7_1015600 heat shock protein 60 (HSP60)
Disruptability [+]
| Species | Disruptability | Reference | Submitter | |
|---|---|---|---|---|
| P. falciparum 3D7 |
Refractory |
USF piggyBac screen (Insert. mut.) | USF PiggyBac Screen | |
| P. berghei ANKA |
Refractory |
PlasmoGEM (Barseq) | PlasmoGEM | |
Mutant phenotypes [+]
None reported yet. Please press the '+' button above to add one.Imaging data (from Malaria Metabolic Pathways)
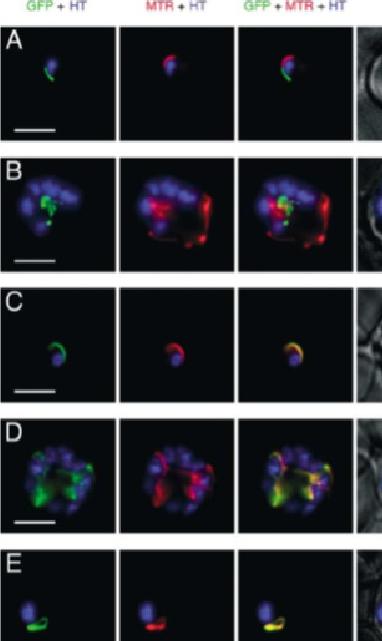
GFP expressed in transfectants of P. falciparum. RBC infected by the transfectant with pSSPF2/PfACP-GFP (A and B), pSSPF2/PfHsp60-GFP (C and D) or pSSPF2/PfIscS-GFP (E and F) were incubated in RPMI 1640 medium containing Hoechst 33342 (HT: blue) at 1 mg/ml and MitoTracker Red CM-H2XRos (MTR: red) at 100nM (A, C, E) or 10nM (B, D, F) to stain the nucleus and mitochondrion, respectively. Localization of GFP (green) in the transfectant parasites in the trophozoite (A, C, E) or schizont (B, D, F) stage was monitored by fluorescence microscopy using a DeltaVison microscope system (Applied Precision). +phase: GFP + MTR + HT superimposed on a phase contrast image. Scale bar: 5 mm. Both Hsp60 and IscS target to the mitochondrion.Sato S, Rangachari K, Wilson RJ. Targeting GFP to the malarial mitochondrion. Mol Biochem Parasitol. 2003 130:155-8 Copyright Elsevier
See original on MMP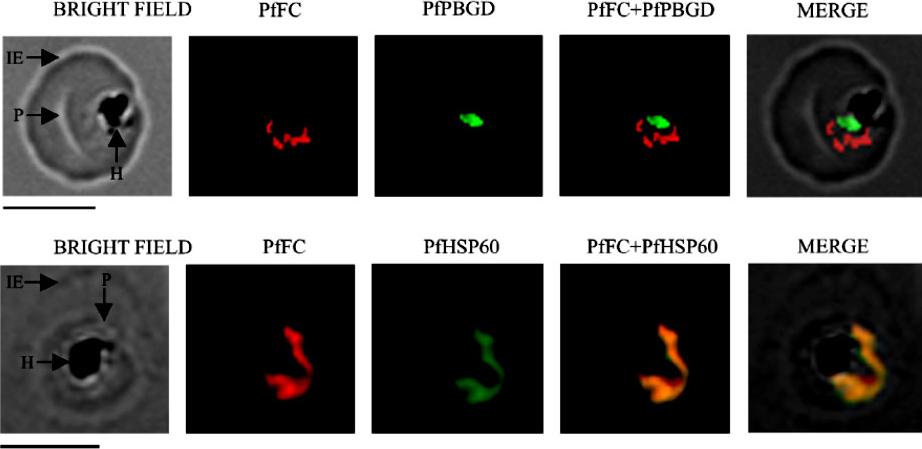
Localization of native, functional PfFC in the parasite by immunofluorescence 304 microscopy. Mouse anti-rPfFC IgG, rabbit anti-r(D)PfPBGD (apicoplast marker) IgG and rabbit anti305 rPfHSP60 (mitochondrial marker) IgG were used at 1:200 dilution. The slides were examined using a 306 Fluorescence Microscope (Leica Microsystems) and the images were acquired at 100x 307 magnification using an oil immersion objective and Leica FW 4000 image acquisition 308 software. IE, infected erythrocyte; P, parasite and H, hemozoin. Scale bar = 5 mm.Nagaraj VA, Prasad D, Rangarajan PN, Padmanaban G. Mitochondrial localization of functional ferrochelatase from Plasmodium falciparum. Mol Biochem Parasitol. 2009 168:109-12. Copyright Elsevier 2010
See original on MMP
Sub-cellular localization of enzymes involved in fumarate to aspartate conversion pathway. Indirect immunofluorescence analysis of parasitized erythrocytes with anti-PfAAT and anti-PfHsp60 antisera. Anti-mouse and anti-rabbit IgG antibodies conjugated with Alexa-Fluor dyes were used as secondary antibodies. Hoechst was used as the nuclear stain. PfAAT is present in the cytosol. No co localization of PfAAT (red) was found with the mitochondrial marker, PfHSP60.Bulusu V, Jayaraman V, Balaram H. Metabolic fate of fumarate, a side product of purine salvage pathway in the intraerythrocytic stages of Plasmodium falciparum. J Biol Chem. 2011 286(11):9236-45.
See original on MMP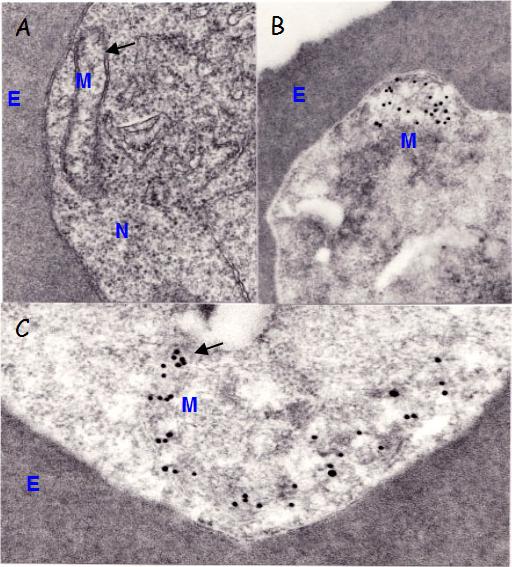
Immunoelectron microscopic localization of PfHSP60. A) Section of trophozoite showing an acristate mitochondrion (M), composed of inner and outer membranes (arrow); E erythrocyte; N- nucleus. B) A mitochondrion is associated with gold particles indicating the localization of Hsp60 molecules. C) Details of a mitochondrion with gold particles. Thin sections of Epon or LR White embedded parasites were reacted first with a-Hsp60 Abs and then with 15 nm gold labeled goat anti-mouse IgG.Das A, Syin C, Fujioka H, Zheng H, Goldman N, Aikawa M, Kumar N. Molecular characterization and ultrastructural localization of Plasmodium falciparum Hsp 60. Mol Biochem Parasitol. 1997 88:95-104. Copyright Elsevier 2009.
See original on MMP
Immunofluorescence images showing multiple localizations of PfTPxGL. PfTPxGL-red signal (anti-mouse Alexa fluor 568), PfPBGD – apicoplast marker, green signal (anti-rabbit FITC), HSP60 – mitochondrial marker, green signal (anti-rabbit FITC). The imaging was carried out with scale bars varying from 5-20 mm The infected RBCs imaged are 6-8 mm across. Parasites showing dual localization of PfTPxGL to the apicoplast and mitochondrionChaudhari R, Narayan A, Patankar S. A novel trafficking pathway in Plasmodium falciparum for the organellar localization of glutathione peroxidase-like thioredoxin peroxidase. FEBS J. 2012 279(20):3872-88
See original on MMP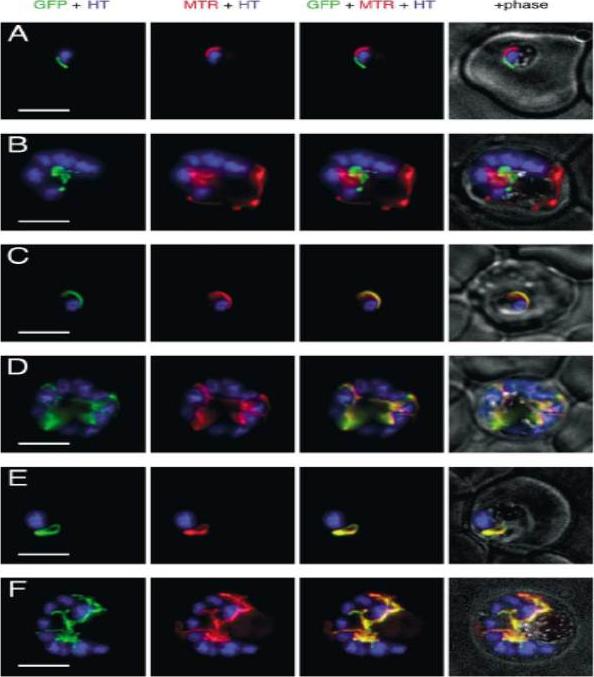
GFP expressed in transfectants of P. falciparum. RBC infected by the transfectant with pSSPF2/PfACP-GFP (A and B), pSSPF2/PfHsp60-GFP (C and D) or pSSPF2/PfIscS-GFP (E and F) were incubated in RPMI 1640 medium containing Hoechst 33342 (HT: blue) at 1 mg/ml and MitoTracker Red CM-H2XRos (MTR: red) at 100nM (A, C, E) or 10nM (B, D, F) to stain the nucleus and mitochondrion, respectively. Localization of GFP (green) in the transfectant parasites in the trophozoite (A, C, E) or schizont (B, D, F) stage was monitored by fluorescence microscopy using a DeltaVison microscope system (Applied Precision). +phase: GFP + MTR + HT superimposed on a phase contrast image. Scale bar: 5 mm. Both Hsp60 and IscS target to the mitochondrion.Sato S, Rangachari K, Wilson RJ. Targeting GFP to the malarial mitochondrion. Mol Biochem Parasitol. 2003 130:155-8.
See original on MMP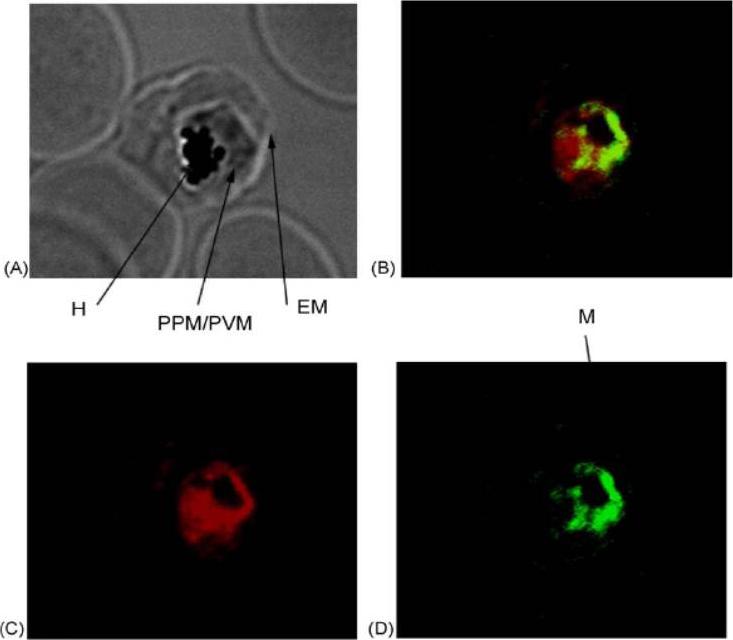
Trophozoite-infected red blood cells were probed with primary antibodies [(rabbit anti-PfIRPa 3950) and (mouse anti-Hsp60)] followed by fluorescent secondary antibodies [(goat anti-rabbit, alexa568) and (goat anti-mouse alexa488)]. The mitochondrial compartment of the intraerythrocytic trophozoite was stained in green (D), whereas PfIRPa was stained in red (C). Light microscopic image of the same trophozoite-infected red blood cell is shown in A. Colocalization of red and green fluorescence (yellow B) is indicative of a mitochondrial localization of PfIRPa, but the protein is also visualized in the parasite cytosol/food vacuole. EM—erythrocyte plasma membrane, PPM—parasite plasma membrane, PVM—parasitophorous vacuole membrane, H—hemozoin, M—mitochondrion.Hodges M, Yikilmaz E, Patterson G, Kasvosve I, Rouault TA, Gordeuk VR, Loyevsky M. An iron regulatory-like protein expressed in Plasmodium falciparum displays aconitase activity. Mol Biochem Parasitol. 2005 143:29-38. Copyright Elsevier 2009.
See original on MMP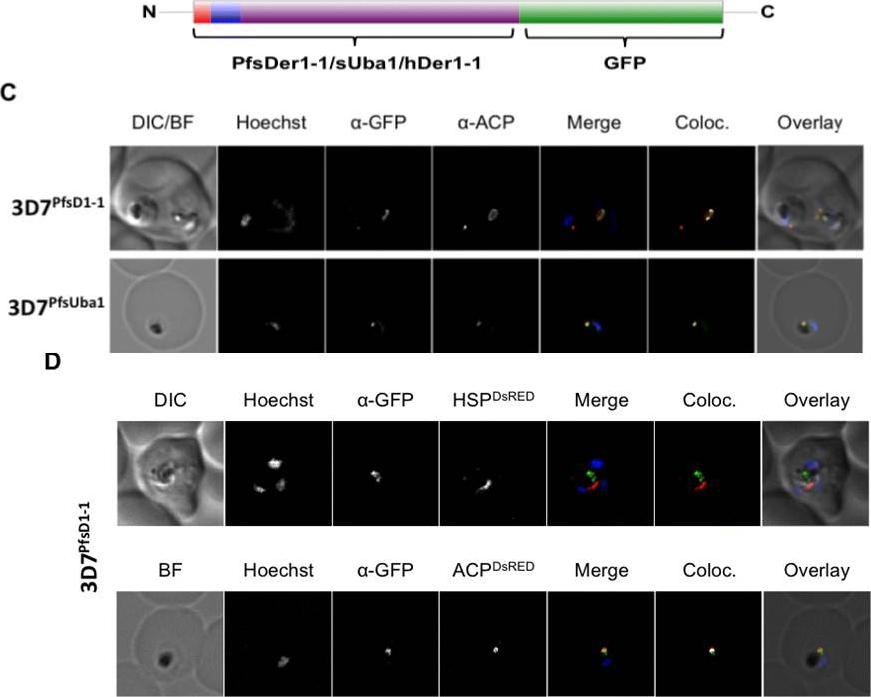
C. In both 3D7PfsD1-1 and 3D7PfsUba1 lines, GFP and ACP signals colocalise, suggesting that both are resident apicoplast proteins. Co-transfected 3D7sD1-1 with a construct encoding either the BTS of the apicoplast resident protein ACP or the mitochondrial targeting sequence of mitochondrial PfHsp60, fused to the red fluorescent protein DsRed. D. Fixed cells co-labelled with anti-GFP antibodies, ACP-DsRed PFB0385w and GFP signals co-localise, whilst HSP60-DsRed and GFP signal do not. Thus, the full-length PfsDer1-1-GFP fusion protein is transported to the apicoplast.Spork S, Hiss JA, Mandel K, Sommer M, Kooij TW, Chu T, Schneider G, Maier UG, Przyborski JM. An unusual ERAD-like complex is targeted to the apicoplast of Plasmodium falciparum. Eukaryot Cell. 2009 ;8(8):1134-45
See original on MMP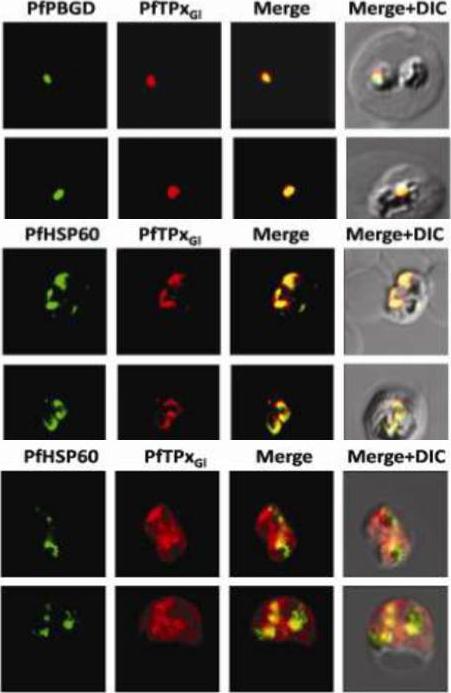
Immunofluorescence images showing multiple localizations of PfTPxGL. PfTPxGL-red signal (anti-mouse Alexa fluor 568), PfPBGD – apicoplast marker, green signal (anti-rabbit FITC), HSP60 – mitochondrial marker, green signal (anti-rabbit FITC). The imaging was carried out with scale bars varying from 5-20 mm. The infected RBCs imaged are 6-8 mm across. (Upper panel) Parasites showing only apicoplast localization of PfTPxGL, (Middle panel) Parasite showing only mitochondrial localization of PfTPxGL, (Lower panel) Parasite showing organellar as well as cytosolic localization of PfTPxGL. Chaudhari R, Narayan A, Patankar S. A novel trafficking pathway in Plasmodium falciparum for the organellar localization of glutathione peroxidase-like thioredoxin peroxidase. FEBS J. 2012 279(20):3872-88.
See original on MMP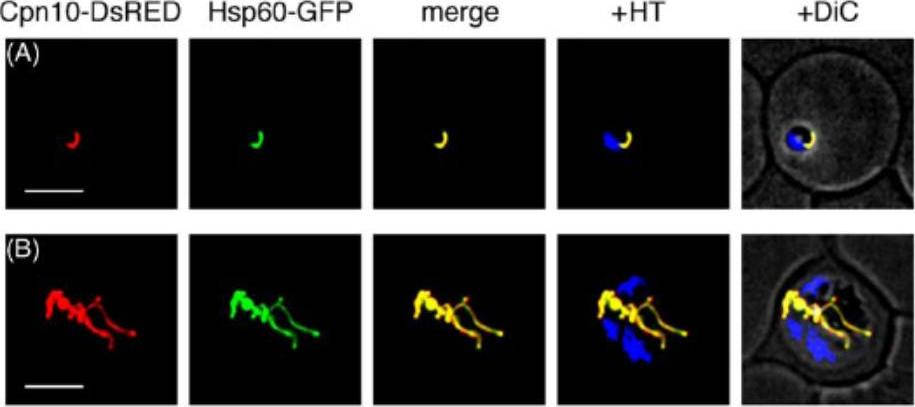
Subcellular localization of fluorescent proteins in transfectants of P. falciparum. Mitochondrial localization of pSSPF2/Pf Cpn10-DsRED and pSSPF2/Pf Hsp60-GFP in a double-transfectant in the ring stage (A) and in the schizont stage (B). Fluorescent proteins (DsRED, red; GFP, green) or MitoTracker Green FM (MTG, green) were monitored by fluorescence microscopy using a DeltaVision microscope system (Applied Precision). +HT, merge with the blue signal of Hoechst 33342 indicating the position of the nucleus of each parasite. +DiC, overlay on the differential phase contrast image. Scale bar: 5 mm.Sato S, Wilson RJ. Organelle-specific cochaperonins in apicomplexan parasites. Mol Biochem Parasitol. 2005 141:133-143. Copyright Elsevier 2009.
See original on MMP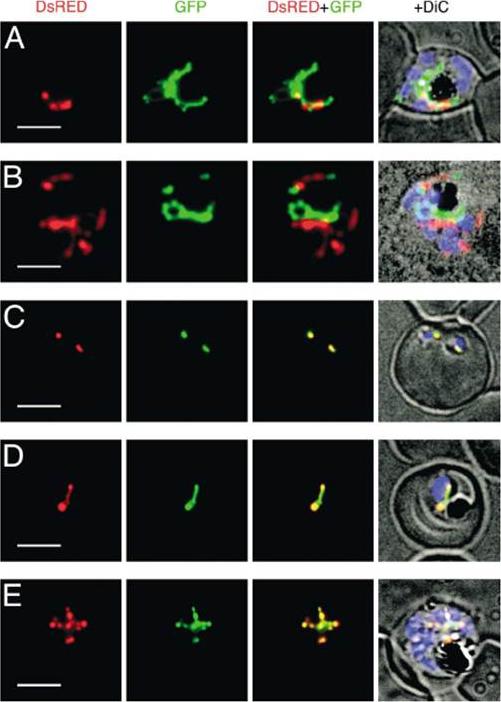
Expression of DsRED and GFP in Plasmodium falciparum double transfectants. Localization of fluorescent proteins DsRED (red) and GFP (green) in pSSPF2/PfHsp60-DsRED–pSSPF2/PfACP-GFP double transfectant (A), pSSPF2/PfACP-DsRED–pSSPF2/PfHsp60-GFP double transfectant (B) and pSSPF2/PfCpn60-DsRED–pSSPF2/PfACP-GFP double transfectants (C–E) was monitored by fluorescence microscopy. +DiC, overlay on a phase contrast image with the blue signal of Hoechst 33342 indicating the position of the nucleus of the parasites. (A, B) Schizont; (C) two co-infecting ring stage parasites; (D) trophozoite; (E) schizont. Scale bar: 5 mm.Sato S, Wilson RJ. The use of DsRED in single- and dual-color fluorescence labeling of mitochondrial and plastid organelles in Plasmodium falciparum. Mol Biochem Parasitol. 2004 134:175-9. Copyright Elsevier
See original on MMP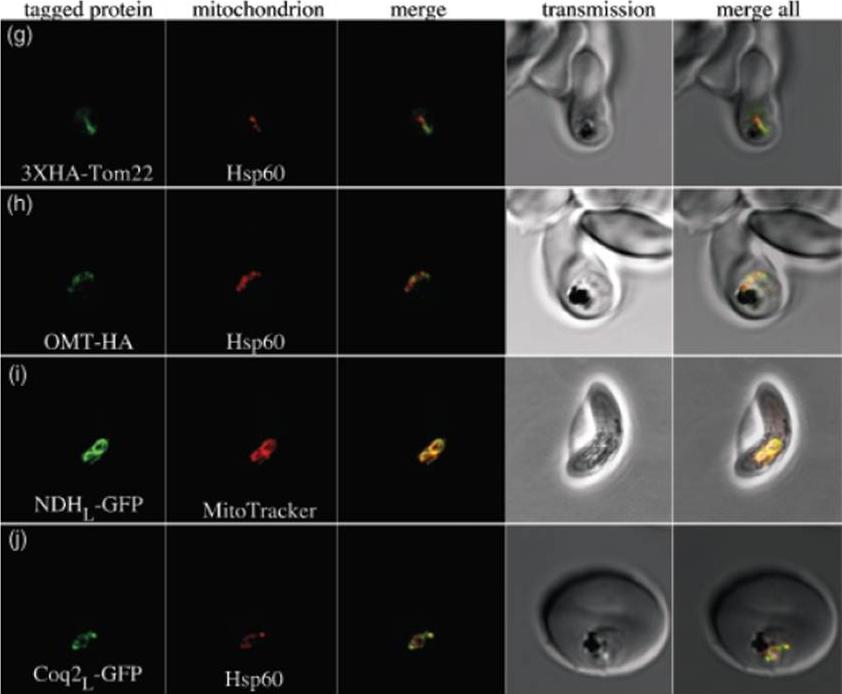
g) PfTom22 localizes to the mitochondrion of P. falciparum. Pf Tom22 was tagged with a 3X-HA epitope tag at its N-terminus. An immuno-fluorescence assay was performed with 3XHA-PfTom22 labelled in green and the mitochondrial marker Hsp60 labelled in red. The merged image (central panel) shows apparent overlap between the two proteins, indicating that PfTom22 is a mitochondrial protein. h) The oxoglutarate/malate transporter homologue of P. falciparum (PfOMT) localizes to the mitochondrion of P. falciparum. PfOMTwas tagged with a 1XHA tag at its C-terminus (green) and co-labelled with anti-Hsp60 antibody (red). The merged image shows apparent overlap between the two proteins, indicating that PfOMT is a mitochondrial protein. i) The single-subunit NAD(P)H dehydrogenase homologue of P. falciparum (PfNDH) localizes to the mitochondrion. The N-terminal extension of PfNDH was fused to green fluorescent protein (GFP, green), with live parasites co-labelled with MitoTracker Red (red), a mitochondrial marker. In this gametocyte image, there is clear overlap, indicating that PfNDH localizes to the mitochondrion. In asexual stages, when protein expression levels are probably higher, a considerable portion of PfNDHL-GFP also localizes to the cytosol, suggesting that import of this fusion protein is somewhat inefficient. j) The coenzyme Q biosynthesis protein Coq2 homologue of P. falciparum (PfCoq2) localizes to the mitochondrion. The N-terminal portion of PfCoq2 was fused to green fluorescent protein and labelled with anti-green fluorescent protein antibody (green). These fixed parasites were co-labelled with anti-Hsp60 antibody (red), with the apparent co-localization indicating that PfCoq2 is a mitochondrial proteinvan Dooren GG, Stimmler LM, McFadden GI. Metabolic maps and functions of the Plasmodium mitochondrion. FEMS Microbiol Rev. 2006 30(4):596-630. Review.
See original on MMP
Co-localization of ALAS with MitoTracker dye and Hsp60 in P. falciparum. Top row : (a) bright ®eld (the contour of the infected cell is indicated since it is not clearly visible) ; (b) Hoechst stain ; (c) MitoTracker fluorescence in parasite ; (d) ALAS immuno¯uorescence with PfALAS antibody and FITC-conjugated secondary antibody ; (e) co-localization of (c) and (d). Bottom row : (a) bright field ; (b) Hoescht stain ; (c) Hsp60 immunofuorescence with Hsp60 antibody in rabbits and tetramethylrhodamine b-isothiocyanate (TRITC)-conjugated anti-rabbit antibody in goat ; (d) ALAS immuno-fluorescence with ALAS antibody in mice and FITC-conjugated anti-mouse antibody in goat ; (e) c-localization of (c) and (d).Varadharajan S, Dhanasekaran S, Bonday ZQ, Rangarajan PN, Padmanaban G. Involvement of delta-aminolaevulinate synthase encoded by the parasite gene in de novo haem synthesis by Plasmodium falciparum. Biochem J. 2002 Oct 15;367(Pt 2):321-7. PubMed
See original on MMP
Sub-cellular distribution and localization of PfHsp60 (continued). (A) IFA of localization PfHsp60 in P. falciparum infected erythrocytes with (i) Propidium iodide (Nuclear marker), (ii) Mitotracker, (iii) PfCytochrome C (Mitochondrial marker), (iv) PfUROD (Apicoplast marker), (v) PfPlasmepsin V (PfPMV, endoplasmic reticulum marker), and (vi) PfHsp70-1 (Cytosol marker). PfHsp60 is stained with FITC-conjugated secondary antibody in all parts, while PfUROD, PfPlasmepsin V, PfCytochrome C, and PfHsp70-1 are stained with TRITC-cojugated secondary antibody in (iii), (iv), and (v). In all images, white arrow indicates the erythrocyte membrane. Part (i) shows that PfHsp60 is present only in the extra-nuclear compartment. Parts (ii) and (iii) show that PfHsp60 is present in the cytoplasm as well as in the mitochondria. The yellow signal in the merged panel shows that there is some co-localization. Part (iv) shows a lack of signal overlap with PfUROD in the merge panel. Part (v) shows a lack of signal overlap with PfPlasmepsin V in the merge panel, which rules out its localization in endoplasmic reticulum. Part (vi) shows that there is co-localization between the cytosolic protein PfHsp70-1 and PfHsp60, which indicates that PfHsp60 indeed accumulates in the cytosolPadma Priya P, Grover M, Tatu US, Natarajan V. Characterization of Precursor PfHsp60 in Plasmodium falciparum Cytosol during Its Asexual Development in Human Erythrocytes. PLoS One. 2015 Aug 28;10(8):e0136401. PMID:
See original on MMP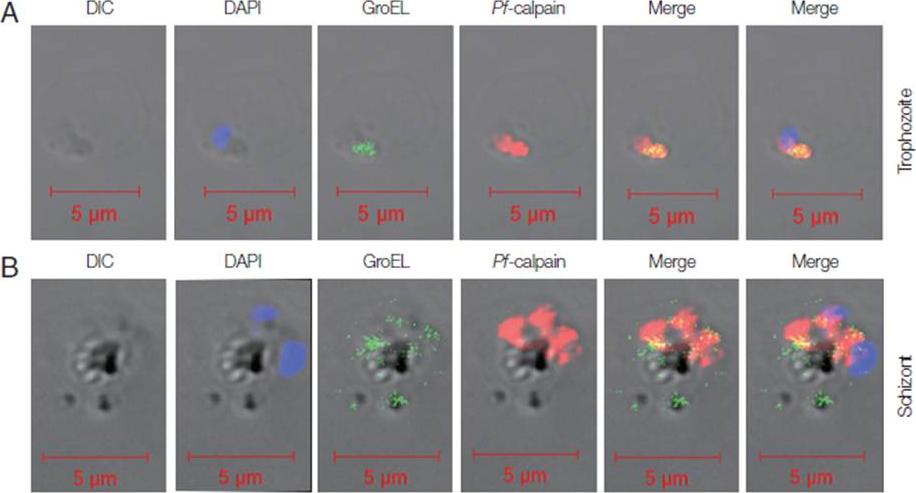
Localization of Pf-Hsp60 and Pf-calpain in the infected RBCs. Co-localization of Pf-Hsp60 and Pf-calpain was observed by IFA. Anti-GroEL-antibody was used for detection of Pf-Hsp60 using FITC-conjugated rabbit-anti-mouse IgG (green). Pf-calpain was visualized by Cy5-conjugated goat-anti-rabbit IgG (red). Pf-Hsp60 was partially co-localized (yellow) with Pf-calpan in both trophozoites (A) and schizonts (B).Yeo SJ, Liu DX, Park H. Potential Interaction of Plasmodium falciparum Hsp60 and Calpain. Korean J Parasitol. 2015 Dec;53(6):665-73.
See original on MMPMore information
| PlasmoDB | PF3D7_1015600 |
| GeneDB | PF3D7_1015600 |
| Malaria Metabolic Pathways | Localisation images Pathways mapped to |
| Previous ID(s) | PF10_0153 |
| Orthologs | PBANKA_1214000 , PCHAS_1214700 , PKNH_0815700 , PVP01_0815700 , PVX_095000 , PY17X_1217200 |
| Google Scholar | Search for all mentions of this gene |