PF3D7_0917900 heat shock protein 70 (HSP70-2)
Disruptability [+]
| Species | Disruptability | Reference | Submitter |
|---|---|---|---|
| P. falciparum 3D7 |
Refractory |
USF piggyBac screen (Insert. mut.) | USF PiggyBac Screen |
Mutant phenotypes [+]
None reported yet. Please press the '+' button above to add one.Imaging data (from Malaria Metabolic Pathways)
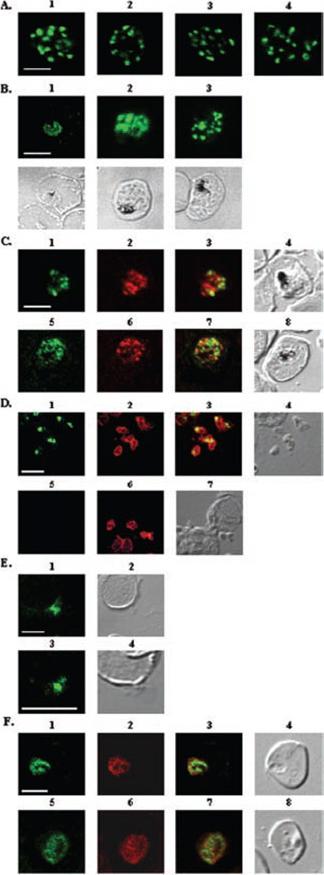
A, localization of RAMA in rhoptry organelles. Confocal microscopy on schizont stage parasites stained with rabbit anti-RAMA-B (1), anti-RAMA-C (2), anti-RAMA-D (3), or anti-RAMA-E (4) sera. B, localization of RAMA in immature parasites. Confocal microscopy on parasites stained with rabbit anti-RAMA-D serum: early trophozoite (1), late trophozoite (2), and early schizont (3) stages. Corresponding differential interference contrast (DIC) images are shown. C, trafficking of RAMA through the secretory pathway. Confocal microscopy on parasites stained with mouse anti-RAMA-E (1 and 5) and either anti-PfGRP (2) or anti-PfERD2 (6) rabbit antibodies. Corresponding overlay (3 and 7) and DIC (4 and 8) images are shown. D, localization of p60/RAMA in free merozoites. Confocal microscopy on parasites stained with mouse anti-MSP4 (2 and 6), and rabbit anti-RAMA-D (1) or anti-RAMA-B (5) sera. Corresponding overlay (3) and DIC (4 and 7) images are shown. E, discharge of RAMA from rhoptries. Confocal microscopy on CytB-treated merozoites incubated with RBCs, stained with rabbit anti-RAMA-D serum (1 and 3). Corresponding DIC images (2 and 4) are shown. F, association with the PV. Confocal microscopy on early ring stage-parasites stained with rabbit anti-RAMA-E serum (1 and 5) and monoclonal anti-RAP1 (2 and 6) antibodies. Corresponding overlay (3 and 7) and DIC (4 and 8) images are shown. Bars in 1 represent 5 mm. RAMA is synthesized as a 170-kDa protein in early trophozoites, several hours before rhoptry formation and is transiently localized within the endoplasmic reticulum and Golgi within lipid-rich microdomains.Topolska AE, Lidgett A, Truman D, Fujioka H, Coppel RL. Characterization of a membrane-associated rhoptry protein of Plasmodium falciparum. J Biol Chem. 2004 279:4648-4656.
See original on MMP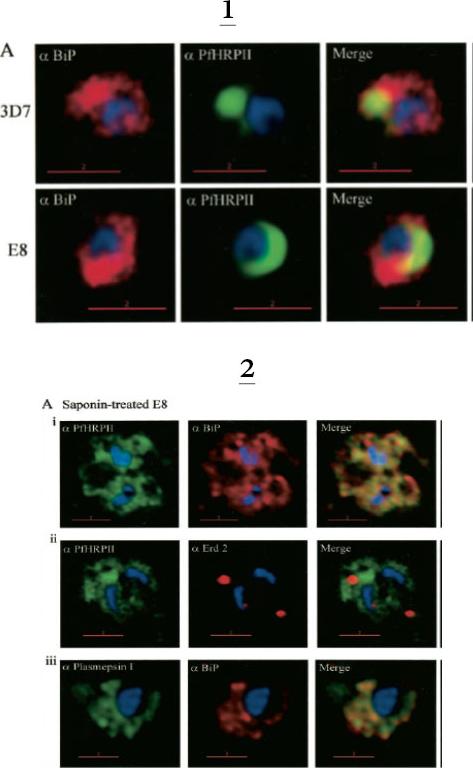
1. Comparative analysis of secretory recruitment of HRPII, export of parasite proteins in E8- and 3D7-infected erythrocytes, and distribution of total PfHRPII, PfHRPIImyc, plasmepsin I, and BiP in parasites and isolate food vacuoles. A, ring-infected red cells from E8 and 3D7-strains were treated with brefeldin A, stained for PfBiP (red), PfHRPII (green), and Hoechst dye (blue) in indirect immunofluorescence assays, and imaged using DeltaVision. Single optical sections showing a merge of red and green as yellow are shown. In the presence of BFA, all cell-associated HRPIIs detected in both E8 and 3D7 reside within regions that stain with the ER marker PfBiP, confirming that HRPII is recruited to the secretory pathway.2. Membrane association of parasite-HRPII in E8 and ACPGFP cells. A, 30–33-h trophozoite-infected red cells from 3D7 and E8 strains were attached to poly-L-lysine-coated cover slips and then permeabilized with 0.01% saponin to release the erythrocytic contents. The adherent cells were then fixed with formaldehyde and probed for the distribution of the indicated proteins by indirect immunofluorescence microscopy. HRPII and plasmepsin I show substantial colocalization with PfBiP in E8 cells but not markers of the Golgi (ERD2).Akompong T, Kadekoppala M, Harrison T, Oksman A, Goldberg DE, Fujioka H, Samuel BU, Sullivan D, Haldar K. Trans expression of a Plasmodium falciparum histidine-rich protein II (HRPII) reveals sorting of soluble proteins in the periphery of the host erythrocyte and disrupts transport to the malarial food vacuole. J Biol Chem. 2002 277:28923-33.
See original on MMP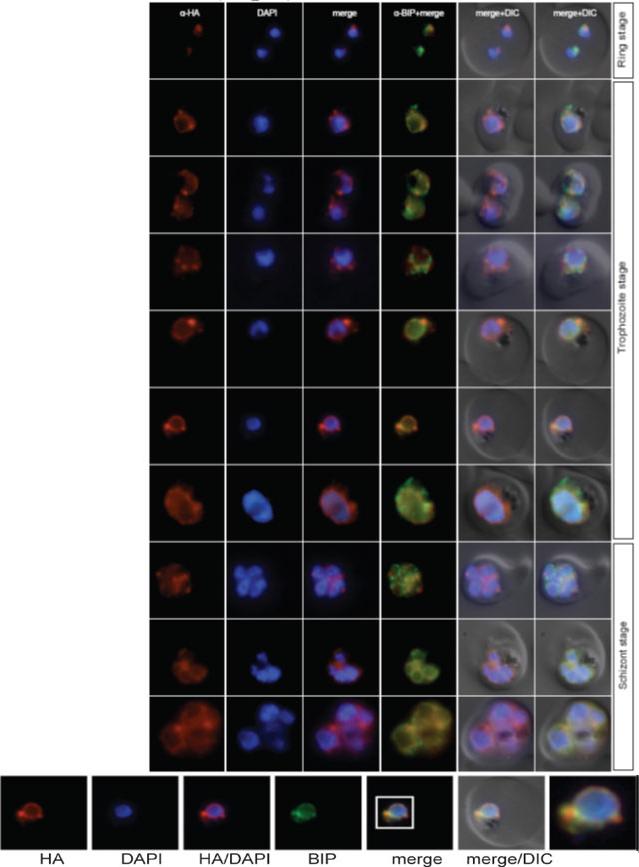
Localisation of n-NuProC4-3xHA (PF10_0100) during the IDC. Localisation of the tagged protein was visualised using anti-HA antibodies (red). Antibodies against PfBIP were used to visualise the ER. DAPI was used to visualise the nucleus. DIC images are shown as reference. The protein is localized either to the ER or to the mitochondrion.Oehring SC, Woodcroft BJ, Moes S, Wetzel J, Dietz O, Pulfer A, Dekiwadia C, Maeser P, Flueck C, Witmer K, Brancucci NM, Niederwieser I, Jenoe P, Ralph SA, Voss TS. Organellar proteomics reveals hundreds of novel nuclear proteins in the malaria parasite Plasmodium falciparum. Genome Biol. 2012 Nov 26;13(11):R108.
See original on MMP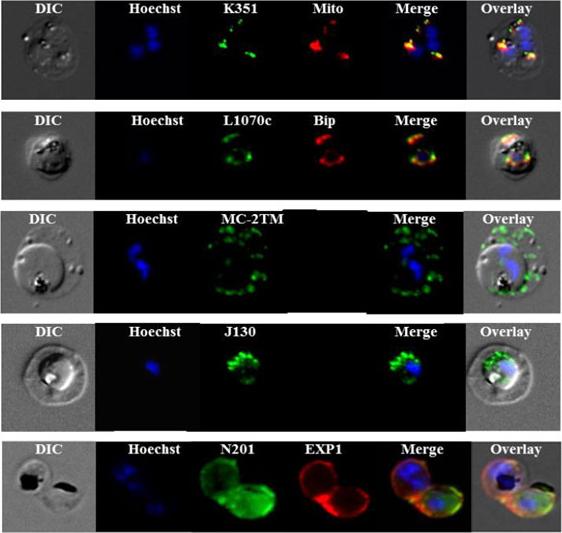
Sub-cellular localization of P. falciparum DRM-associated proteins. Immunofluorescence studies were performed on paraformaldehyde-fixed P.falciparum trophozoite-iRBCs using mouse immune sera raised against the DRM-associated proteins PFJ130, (PF10_0130) PFN201, (PF14_0201 surface protein) PFB985c, (PFB0985c) Pfmc-2TM Maurer's cleft two transmembrane protein; PFL1070c, endoplasmin homolog precursor) PFK351 (PF11_0351) HSP70. Immune sera specific for PfBip and PfEXP1-were used for co-labeling, as indicated. Mitochondria labeling was performed with MitoTracker® probe. Parasite nuclei were stained with Hoechst 33342. Microscopic observations were made on an apotome-equipped Axio Imager 2 (Carl Ziess, Jena) with a 100× apochromat objective and DIC. The differential interference contrast (DIC), merged and overlay images are presented. Bar size: 5 µm. PfK351overlays mitotracker thus localized to parasite mitochondrion. PfL1070 colocalizes with the ER-resident marker PfBiP. PfK55, a conserved hypothetical protein with unknown function, contains a SP and a ER retention signal (KDEL), but due to poor IFA, the exact subcellular localization of this protein remained elusive.Yam XY, Birago C, Fratini F, Di Girolamo F, Raggi C, Sargiacomo M, Bachi A, Berry L, Fall G, Curra C, Pizzi E, Braun-Breton C, Ponzi M. Proteomic analysis of detergent-resistant membrane microdomains in trophozoite blood stage of the human malaria parasite Plasmodium falciparum. Mol Cell Proteomics. 2013 12(12):3948-61
See original on MMP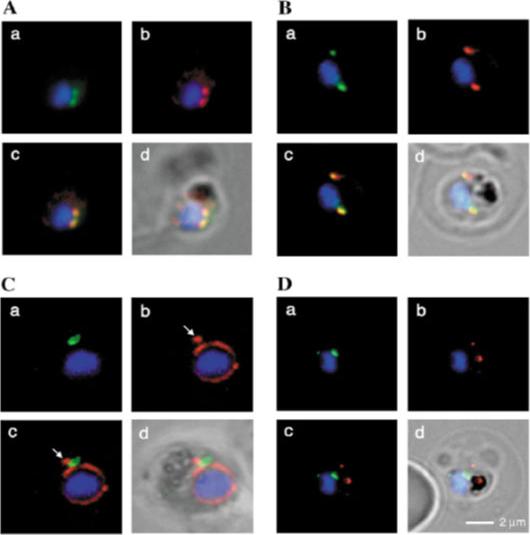
Fluorescence microscopy on fixed parasites. (A) PfGRASP-GFP colocalises with antiPfGRASP-specific antibodies. PfGRASP-GFP is tightly confined to two compartments (a, green) near the parasite nucleus (a, blue). AntiPfGRASP-specific antibodies show a similar staining pattern (b, red with nucleus in blue). Merged image shows identical colocalisation. (B) PfGRASP-GFP colocalises with the cis-Golgi marker ERD2. PfGRASP-GFP (a, green) accumulates in two discrete compartments in close proximity to the nucleus (a, blue). Anti-PfERD2 antibodies recognize similar structures (b, red with nucleus in blue). Merged image shows colocalisation of compartments (c, yellow). (C) PfGRASP-GFP defines a compartment that is distinct from the ER. At the early stages of the parasite life cycle (<16 hours post invasion) PfGRASP is restricted to one compartment (a, green) juxtapose to the nucleus (a, blue). The ER is visualised by anti-PfBiP-specific antibodies (b, red). The membranous system of the ER forms an envelope around the nucleus (b, blue) with one protrusion (indicated by arrow). Merged image shows no colocalisation of the two compartments (c). (D) PfGRASP-GFP does not colocalise with the trans-Golgi marker PfRab6. PfGRASP accumulates in two discrete foci (a, green) adjacent to the nucleus (a, blue). Antibodies against PfRab6 visualise two distinct sites within the parasite (b, red with nucleus in blue). Merged image shows no colocalisation of the PfGRASP defined compartment with PfRab6 (c).Struck NS, de Souza Dias S, Langer C, Marti M, Pearce JA, Cowman AF, Gilberger TW. Re-defining the Golgi complex in Plasmodium falciparum using the novel Golgi marker PfGRASP. J Cell Sci. 2005 118(Pt 23):5603-13.
See original on MMP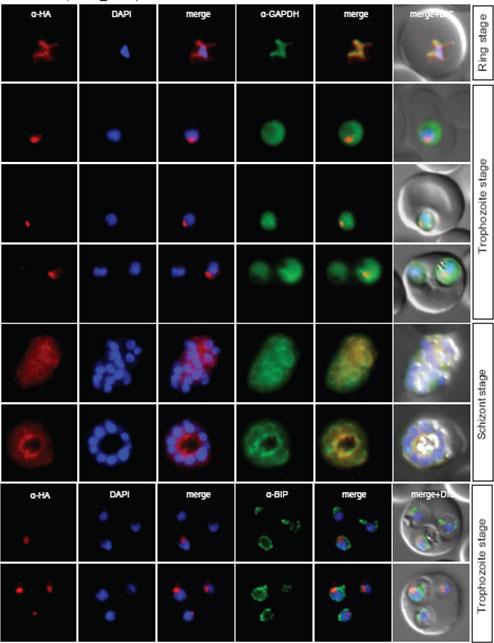
Localisation of NuProC2-3xHA (PF10_0278) during the IDC. Localisation of the tagged protein was visualised using anti-HA antibodies (red). Antibodies against GAPDH and PfBIP were used to visualise the cytosolic (top panel) and ER (bottom panel) compartments, respectively. DAPI was used to visualise the nucleus. DIC images are shown as reference. The protein co-localized with fibrillarin to the parasite nucleolus.Oehring SC, Woodcroft BJ, Moes S, Wetzel J, Dietz O, Pulfer A, Dekiwadia C, Maeser P, Flueck C, Witmer K, Brancucci NM, Niederwieser I, Jenoe P, Ralph SA, Voss TS. Organellar proteomics reveals hundreds of novel nuclear proteins in the malaria parasite Plasmodium falciparum. Genome Biol. 2012 Nov 26;13(11):R108.
See original on MMP
Localisation of PF11_0099 during the IDC. Localisation of the tagged protein was visualised using anti-HA antibodies (red). Antibodies against PfBIP were used to visualise the ER. DAPI was used to visualise the nucleus. DIC images are shown as reference. PF11_0099 shows a ER-like staining throughout the IDC (very clear co-localisation with BIP in rings and trophs, a bit less obvious in schizonts). Oehring SC, Woodcroft BJ, Moes S, Wetzel J, Dietz O, Pulfer A, Dekiwadia C, Maeser P, Flueck C, Witmer K, Brancucci NM, Niederwieser I, Jenoe P, Ralph SA, Voss TS. Organellar proteomics reveals hundreds of novel nuclear proteins in the malaria parasite Plasmodium falciparum. Genome Biol. 2012 Nov 26;13(11):R108.
See original on MMP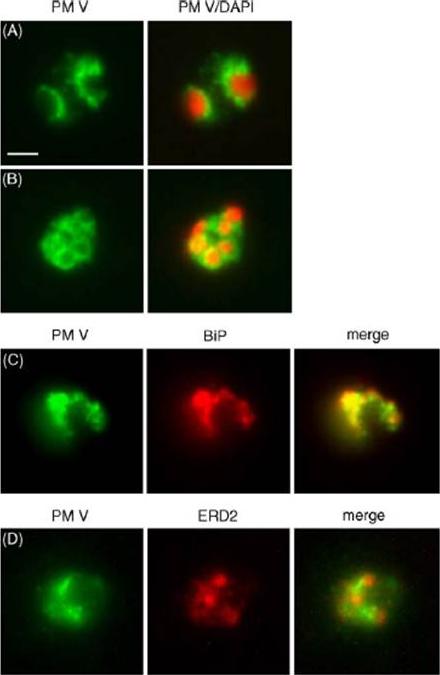
Localization of plasmepsin V (PM V). Indirect immunofluorescence assays of PM V (green) in trophozoites (A) and a ~6N schizont (B) in formaldehyde-fixed blood smears. Nuclei were visualized with DAPI (pseudocolored red). (C, D) Colocalization of PM V and the ER protein BiP (C) or the Golgi marker ERD2 (D) in trophozoites. Bar, 2 mm. In trophozoites and schizonts, PM V was concentrated around the nucleus with lower levels dispersed in the surrounding cytoplasm (Fig. 5A and B). These patterns of fluorescence suggest that PM V resides in the endoplasmic reticulum (ER), which in P. falciparum has been shown to include the nuclear envelope.Klemba M, Goldberg DE. Characterization of plasmepsin V, a membrane-bound aspartic protease homolog in the endoplasmic reticulum of Plasmodium falciparum. Mol Biochem Parasitol. 2005 143:183-191. Copyright Elsevier 2010.
See original on MMP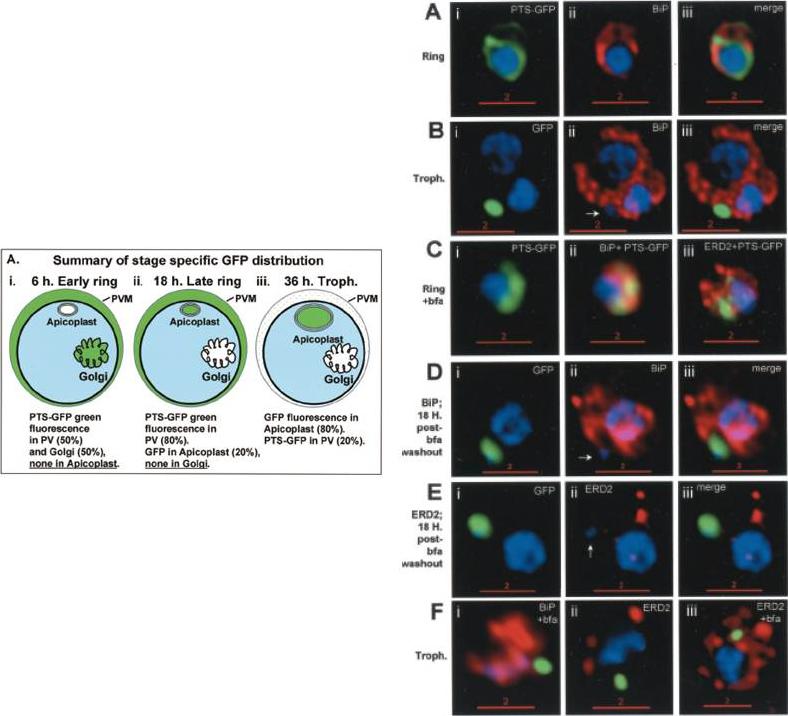
Stage-specific localization of apicoplast-targeted GFP and the effects of brefeldin A (bfa). Distribution of green fluorescence and PfBiP (red) in a early-ring (A, i–iii) and a trophozoite (B, i–iii; a late, 33 h, trophozoite with two nuclei stained in blue is shown). Distribution of green fluorescence and indicated secretory marker (red) in: rings incubated with Bfa for 24 h (C, i–iii); rings incubated with Bfa for 24 h, washed, and grown for 18 h in absence of drug (D, i–iii; E, i–iii); and trophozoites incubated with or without Bfa (F, i–iii). Blue indicates DNA stained with Hoechst 33342. White arrows in B, ii, D, ii, and E, ii, indicate apicoplast DNA. Scale bars as indicated in microns.Cheresh P, Harrison T, Fujioka H, Haldar K. Targeting the malarial plastid via the parasitophorous vacuole. J Biol Chem. 2002 277:16265-77.
See original on MMP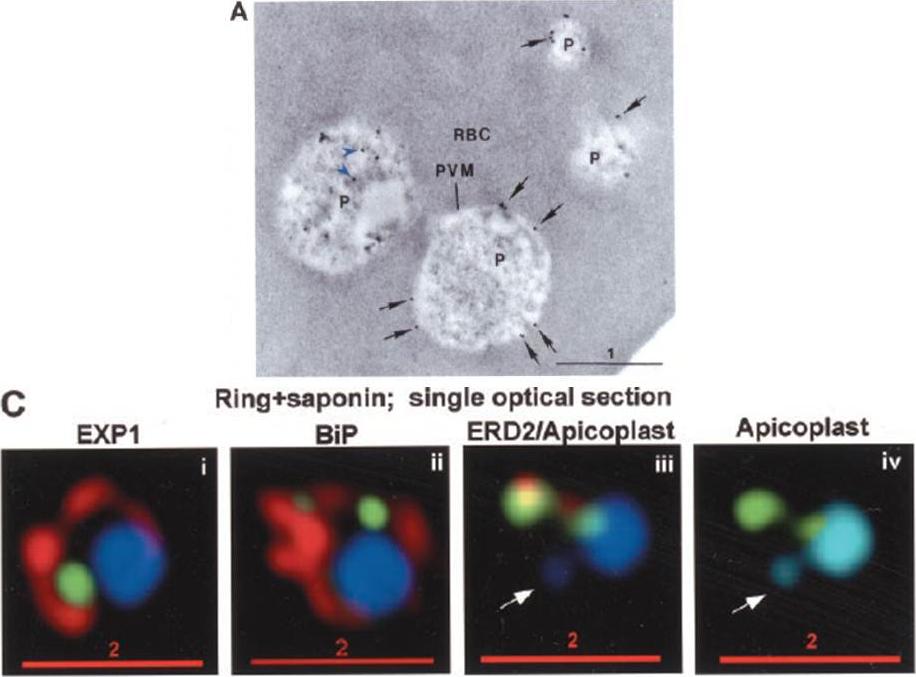
PTS-GFP is detected in the parasitophorous vacuole and in apposition with the Golgi in early rings. A, immunoelectron micrograph of rings showing localization of PTS (plastid-targeting-signal)-GFP in the parasitophorous vacuole and PVM (black arrows) as well as within the parasite (P; blue arrowheads). RBC, red blood cell. Scale bar, 1 mm.. C, i–iv: single optical sections showing green fluorescence in young rings permeabilized with 0.01% saponin relative to secretory markers PfEXP1, PfBiP, PfERD2 (shown in red in i–iii), and apicoplast DNA (marked with an arrow in iii and iv), as detected by indirect immunofluorescence and DeltaVision Microscopy. In C, iv, the Hoechst stain is pseudo-colored cyan to facilitate visualization of apicoplast DNA.Cheresh P, Harrison T, Fujioka H, Haldar K. Targeting the malarial plastid via the parasitophorous vacuole. J Biol Chem. 2002 277:16265-77.
See original on MMP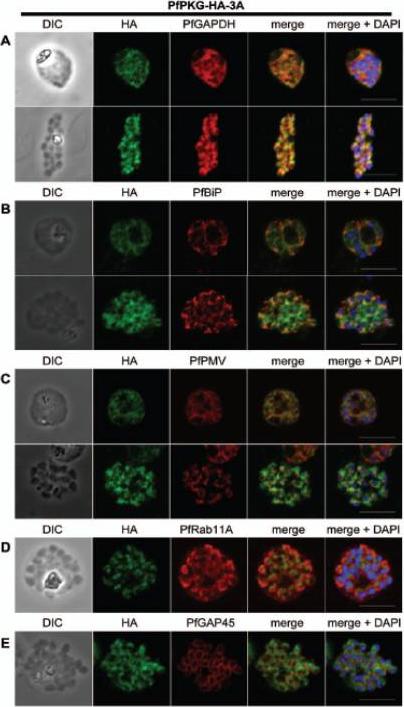
Subcellular location of PfPKG in mature schizonts. Dual immunofluorescent detection of PfPKG-HA in fixed smears of early and late schizonts of the PfPKG-HA-3A clone together with (A) GAPDH; (B) BiP; (C) PMV; (D) Rab11A and (E) GAP45. Representative images are shown for each antibody, together with bright field images (first column) and parasite nuclei stained with DAPI (in the merged image). Bars ,5 mm. Significant overlap between the PKG-HA and GAPDH in early and late blood stage schizonts (A), both are in the cytosol. PfPKG-HA also partially overlapped with that of the ER membrane protein PMV and that of the ER lumen marker PfBiP (B and C) in both early and late schizonts. Rab11A is thought to be involved in vesicle trafficking and localises to the rhoptries and the inner membrane complex (IMC) at the apical end of the merozoite. In mature schizonts, the location of PKG-HA appeared to be largely distinct from that of Rab11A (D). PKG-HA appeared to be absent from the IMC in segmented schizonts, since it did not colocalise with the IMC marker, the glideosome associated protein 45. Hopp CS, Flueck C, Solyakov L, Tobin A, Baker DA. Spatiotemporal and Functional Characterisation of the Plasmodium falciparum cGMP-Dependent Protein Kinase. PLoS One. 2012;7(11):e48206.
See original on MMP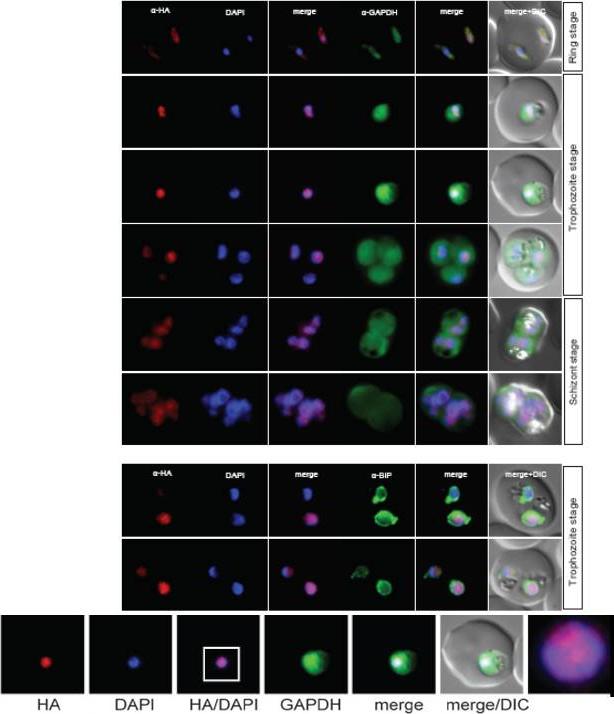
Localisation of PF14_0393 during the IDC. Localisation of the tagged protein was visualised using anti-HA antibodies (red). Antibodies against GAPDH were used to visualise the cytosolic compartment. Antibodies against PfBIP were used to visualise the ER (bottom). DAPI was used to visualise the nucleus. DIC images are shown as reference. The protein displayed a condensed appearance in the nucleus throughout the IDC. Oehring SC, Woodcroft BJ, Moes S, Wetzel J, Dietz O, Pulfer A, Dekiwadia C, Maeser P, Flueck C, Witmer K, Brancucci NM, Niederwieser I, Jenoe P, Ralph SA, Voss TS. Organellar proteomics reveals hundreds of novel nuclear proteins in the malaria parasite Plasmodium falciparum. Genome Biol. 2012 13(11):R108.
See original on MMP
PfSPP localises to the parasite nuclear periphery. IFA of ring, trophozite and schizont stages of PfSPP-HA labeled with anti-PfERC and HA antibodies. Scale bar 1 μm. Co-labeling with ER markers BiP and ER-calcium binding protein (ERC) by IFA showed strong coincidental labeling with PfSPP fluorescence across the entire intra-erythrocytic development of the parasite. SPP is localized to the ER.Marapana DS, Wilson DW, Zuccala ES, Dekiwadia CD, Beeson JG, Ralph SA, Baum J. Malaria parasite signal peptide peptidase is an ER-resident protease required for growth but not invasion. Traffic. 2012 13(11):1457-65
See original on MMP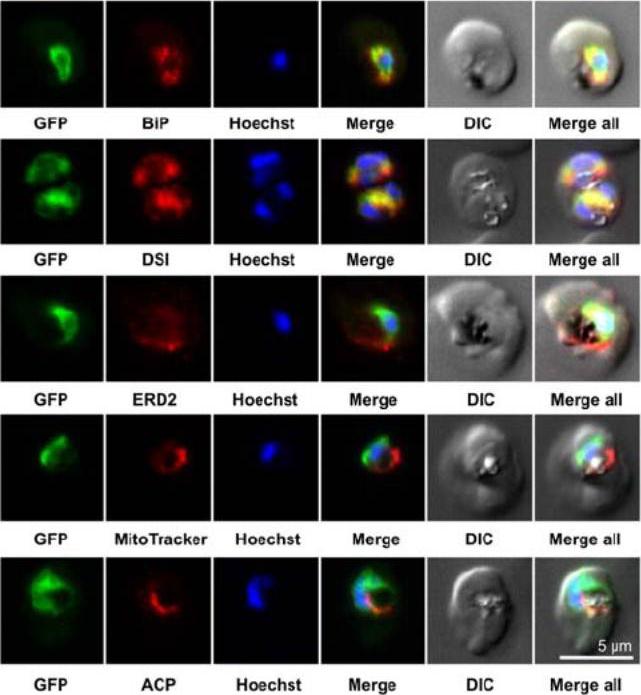
Colocalization studies. PfSCD-GFP was colocalized with different compartment markers: ER (PfBip, PfDSI), cis-Golgi (PfERD2), mitochondria (MitoTracker Red), and apicoplast (PfACP PFB0385w). PfSCD-GFP colocalized mostly with PfBiP and PfDSI (disulfide isomerase), two soluble ER markers, therefore confirming the ER localization of PfSCD-GFP. In contrast, the GFP-tagged PfSCD protein did not colocalize with ERD2, a known marker of the cis-Golgi.Gratraud P, Huws E, Falkard B, Adjalley S, Fidock DA, Berry L, Jacobs WR Jr, Baird MS, Vial H, Kremer L. Oleic acid biosynthesis in Plasmodium falciparum: characterization of the stearoyl-CoA desaturase and investigation as a potential therapeutic target. PLoS One. 2009 4(9):e6889.
See original on MMP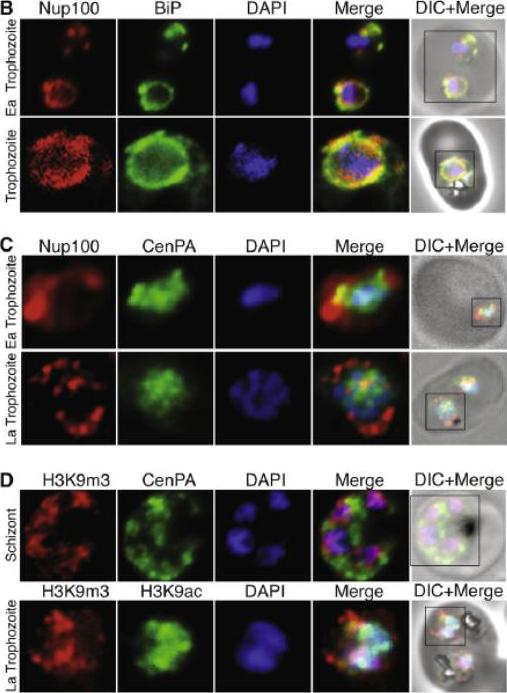
(B) Nucleoporin 100 (Nup100), a protein of the nuclear pore and BiP immunofluorescence analysis reveals the localization of Nup100 outside the DAPI stained area. Nup100 localizes in close proximity to BiP, a marker for the endoplasmic reticulum (ER) and does not overlap with DAPI, confirming that it defines the outer limits of the nucleus. (C) Localization of Nup100 and CenPA (histone 3) outside the DAPI stained area. CenPA and Nup100 do not co-localize; however, CenPA extends beyond the DAPI staining area of the nucleus suggesting that it is located within the periphery but inside the Nup100 delimited area of the nucleus (D) Localization of the histone marks H3K9m3 with CenPA and H3K9ac in the nucleus. The histone modification H3K9m3 silences transcription whereas H3K9ac is found associated with active transcription in general. H3K9m3 localized mostly to the nuclear periphery outside the DAPI stained area, which was consistent with the location of chromosome clusters containing silenced var genes. H3K9ac localized more with the DAPI stained area of the nucleus suggesting the DAPI stained area is broadly the transcriptionally active zone.Ea Trophozoite, Early Trophozoite; La trophozoite, Late Trophozoite.Volz J, Carvalho TG, Ralph SA, Gilson P, Thompson J, Tonkin CJ, Langer C, Crabb BS, Cowman AF. Potential epigenetic regulatory proteins localise to distinct nuclear sub-compartments in Plasmodium falciparum. Int J Parasitol. 2010 40:109-21. Copyright Elsevier 2010.
See original on MMP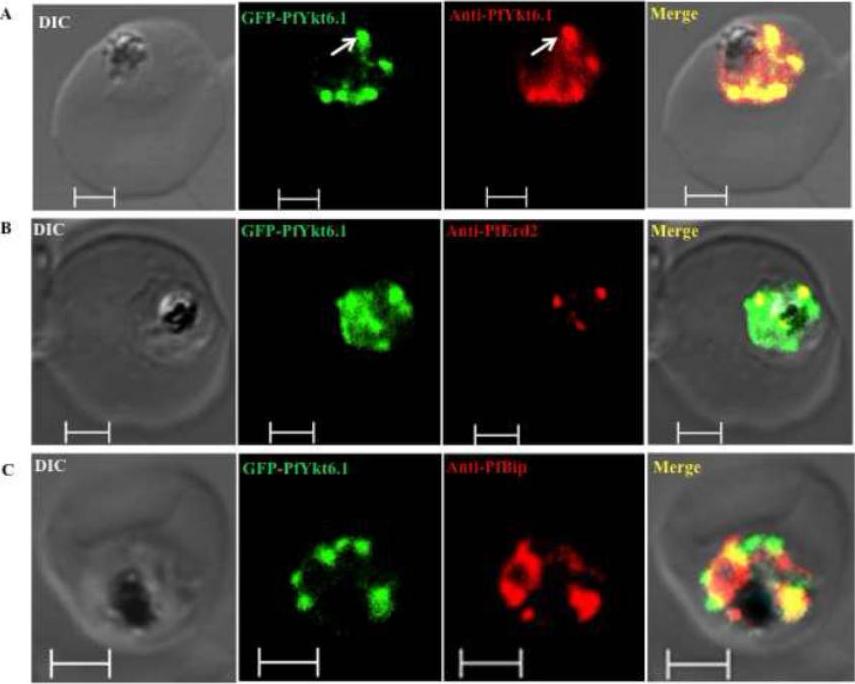
Compartmentalization of PfYkt6.1 in P. falciparum parasites. Trophozoite stage parasites exhibiting increased targeting of the PfYkt6.1 protein to organellar compartments were by confocal immunofluorescence microscopy using various organellar markers. (A) Immunofluorescence micrograph showing complete overlap between the GFP fluorescence and anti-PfYkt6.1 antibody signal in GFP-PfYkt6.1 expressing parasites. The GFP-PfYkt6.1 compartments were only partially labeled with antibodies against the Golgi marker PfErd2 (B), or the ER marker PfBip (C). Scale bars, 2μM. PfYkt6.1 appeared to be partly cytosolic and partly membrane-associated inside the parasite cytoplasm,Ayong L, Dasilva T, Mauser J, Allen CM, Chakrabarti D. Evidence for Prenylation-Dependent Targeting of a Ykt6 SNARE in Plasmodium falciparum. Mol Biochem Parasitol. 2010 175(2):162-8. Copyright Elsevier 2011.
See original on MMP
Parasite sections were incubated with rabbit anti-BiP serum and then labelled with anti-rabbit gold-conjugated IgG. (A) Untreated trophozoite stage showing gold particles lining the endoplasmic reticulum and the nuclear envelope. Note the connection between these organelles. N, nucleus; P, pigmented digestive vesicle; (*), endoplasmic reticulum. Scale bar represents 0.5 mm. (B) Brefeldin A-treated trophozoite stage. Compared with the control, the Brefeldin A-treated cells present a swollen endoplasmic reticulum. The inset shows the intense labelling of a cisternum with gold particles.Nacer A, Berry L, Slomianny C, Mattei D. Plasmodium falciparum signal sequences: simply sequences or special signals? Int J Parasitol. 2001 31:1371-9. Copyright Elsevier
See original on MMP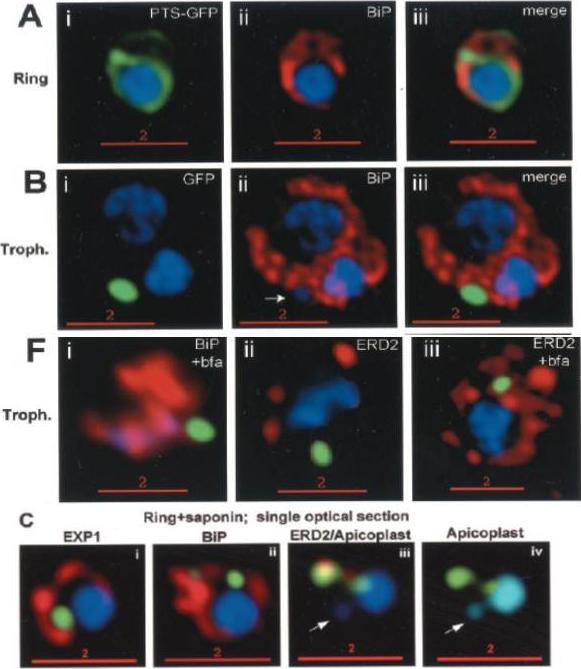
Stage-specific localization of apicoplast-targeted GFP. Distribution of green fluorescence and PfBiP (red) in a early-ring (A, i–iii) and a trophozoite (B, i–iii; a late, ~33 h, trophozoite with two nuclei stained in blue is shown). Distribution of green fluorescence and indicated secretory marker (red) in trophozoites incubated. Blue indicates DNA stained with Hoechst 33342. White arrows in B, ii, D, ii, and E, ii, indicate apicoplast DNA. Scale bars as indicated in microns. C, i–iv: single optical sections showing green fluorescence in young rings permeabilized with 0.01% saponin relative to secretory markers PfEXP1, PfBiP, PfERD2 (shown in red in i–iii), and apicoplast DNA (marked with an arrow in iii and iv), as detected by indirect. In C, iv, the Hoechst stain is pseudo-colored cyan to facilitate visualization of apicoplast DNA.Cheresh P, Harrison T, Fujioka H, Haldar K. Targeting the malarial plastid via the parasitophorous vacuole. J Biol Chem. 2002 277:16265-77.
See original on MMP
Confocal fluorescence microscopy images of transfected 3D7 P. falciparum-infected RBCs expressing a GFP chimera of PfBiP (GRP78) directed to the endoplasmic reticulum. DIC image, the GFP fluorescence signal and an overlay of a P. falciparum immunoglobin binding protein-GFP. Scale bar = 5 µm. In the ring and trophozoite stages (top and middle rows), the endoplasmic reticulum appears as a ring around the nucleus. In schizont stage parasites fluorescence is observed around the nucleus of individual merozoites (daughter cells).van Dooren GG, Marti M, Tonkin CJ, Stimmler LM, Cowman AF, McFadden GI. Development of the endoplasmic reticulum, mitochondrion and apicoplast during the asexual life cycle of Plasmodium falciparum. Mol Microbiol. 2005 57:405-19
See original on MMP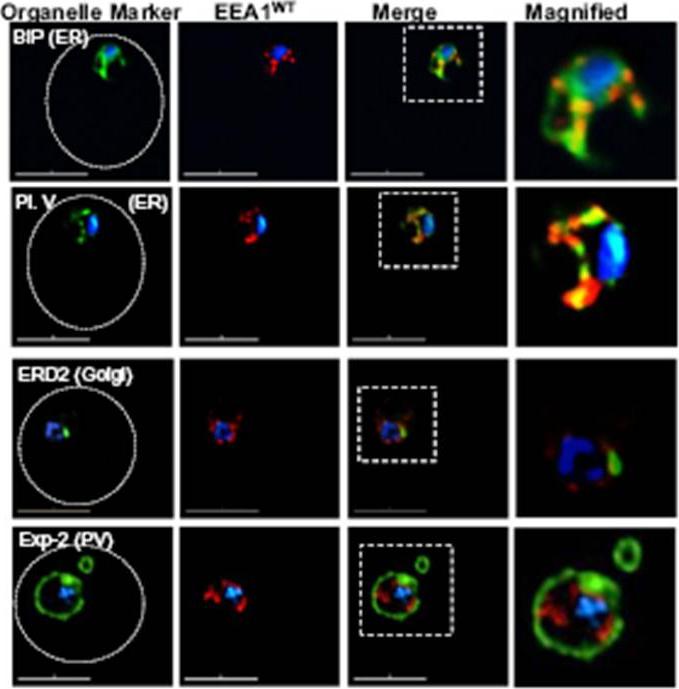
Localization of EEA1WT-mCherry as a marker for PI(3)P (phosphatidylinositol-3-phosphate) in the parasite ER. EEA1 = early endosomal antigen 1. Transgenic parasites transfected with EEA1 were fixed, permeabilized and probed with anti-mCherry as well as antibodies to endogenous P. falciparum markers (green) of the ER, like BIP (top row) and plasmepsin V (Pl. V, second row); a marker of the Golgi (ERD2, third row) or the PVM (Exp-2, bottom row). EEA1WT (red) is seen in punctuate ‘spots’ within the ER. Dotted circles show location of red cell membrane. Dotted squares show regions magnified in the right panels. Parasite nuclei were stained with Hoechst 33342 (blue). Scale bars, 5 mm. The overlap between markers and EEA1 indicates the presence of PI(3)P in the respective regions.Bhattacharjee S, Stahelin RV, Speicher KD, Speicher DW, Haldar K. Endoplasmic Reticulum PI(3)P Lipid Binding Targets Malaria Proteins to the Host Cell. Cell. 2012 148(1-2):201-12.
See original on MMP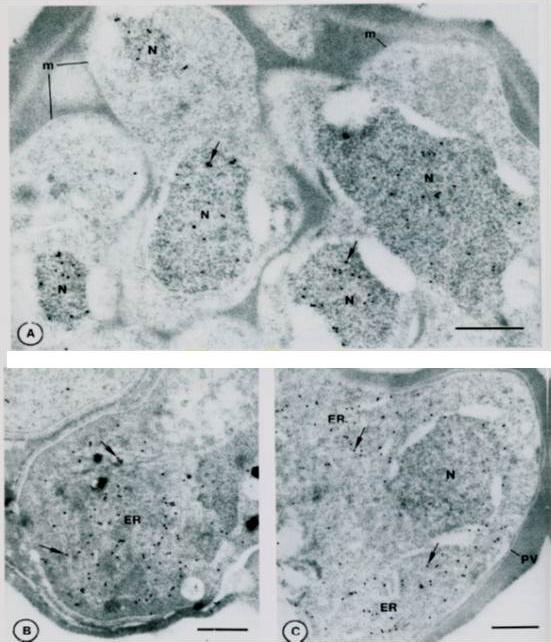
Immuno-gold localization of Pfhsp and Pfgrp. A. Sections of a mature segmenter with rappit antiserum against Pfhsp fusion protein and protein A-gold. The nuclei (N) of budding merozoites (m) are labeled diffusely with colloidal gold particles (arrows). B. Section of mature gametocyte incubated witrh antiserum against Pfgrp peptide and protein A-gold.. Label (arrows) is associated with membranes of the ER. C. Sections of a schizont. Lable (arrows) is associated with discrete areas of the schizont cytoplasm that correspond to ER. Not absence of label on the nucleus (N) and parasitophorous vacuole (PV). Bar – 0.5 mm.Kumar N, Koski G, Harada M, Aikawa M, Zheng H. Induction and localization of Plasmodium falciparum stress proteins related to the heat shock protein 70 family. Mol Biochem Parasitol. 1991 48:47-58. Copyright Elsevier 2009.
See original on MMP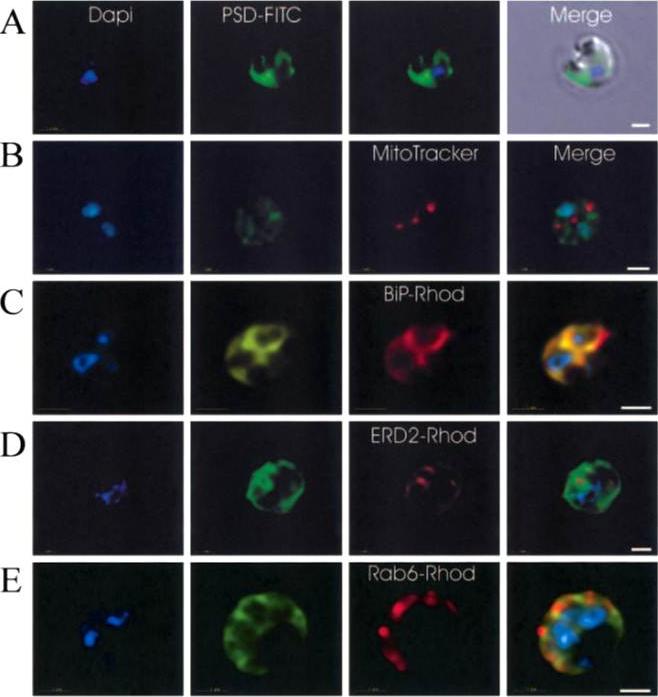
Immunofluorescence localization of PfPSD within P. falciparum-infected erythrocytes. A. FITC (which labels PfPSD) and DAPI (which labels nuclei) images were merged with the Nomarsky image to show the location of PSD labelling in the parasite. DAPI and FITC images were merged with the rhodamine channel corresponding to MitoTracker that labels the mitochondrion (B), BiP-rhodamine that labels the ER (C), ERD2-rhodamine that labels the cis-Golgi (D) or Rab6 -rhodamine that labels the trans-Golgi (E). All the images except (A) correspond to one selected z-section image after digital deconvolution. The bar corresponds to 1 mm. The entire PfPSD labelling was clearly co-localized with the BiP endoplasmic reticulum marker.Baunaure F, Eldin P, Cathiard AM, Vial H. characterization of a non-mitochondrial type I phosphatidylserine decarboxylase in Plasmodium falciparum. Mol Microbiol. 2004 51:33-46. Copyright John Wiley & Sons Ltd. 2010.
See original on MMP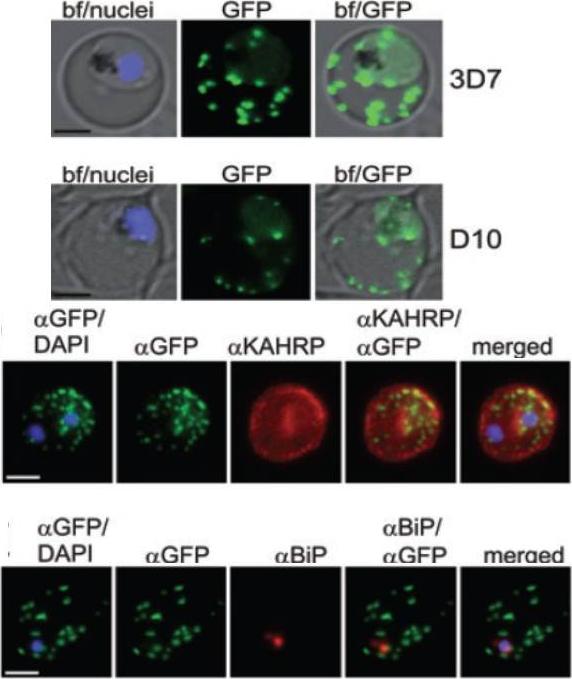
A and B. GFP fluorescence in live 3D7 (A) or D10 (B) parasites expressing wild-type REX2GFP from an episomal vector. GFP fluorescence appears as dots in the host cell cytoplasm typical for Maurer’s cleft localization. Nuclei are stained with DAPI (blue); merges with bright field images (bf) are shown. D. IFA of acetone-fixed infected RBCs expressing REX2GFP and stained with anti-GFP (green) and anti-KAHRP (red) antibodies. Overlays are shown as indicated above each panel. The last panel shows an overlay of all three images (merged). E. IFA of acetone-fixed infected RBCs expressing REX2GFP and stained with anti-GFP (green) and anti-BiP (red) antibodies. Overlays of panels are shown as in (D). Size bars are 2 mm.Haase S, Herrmann S, Grüring C, Heiber A, Jansen PW, Langer C, Treeck M, Cabrera A, Bruns C, Struck NS, Kono M, Engelberg K, Ruch U, Stunnenberg HG, Gilberger TW, Spielmann T. Sequence requirements for the export of the Plasmodium falciparum Maurer's clefts protein REX2. Mol Microbiol. 2009 71:1003-17. Copyright John Wiley & Sons Ltd. 2010.
See original on MMP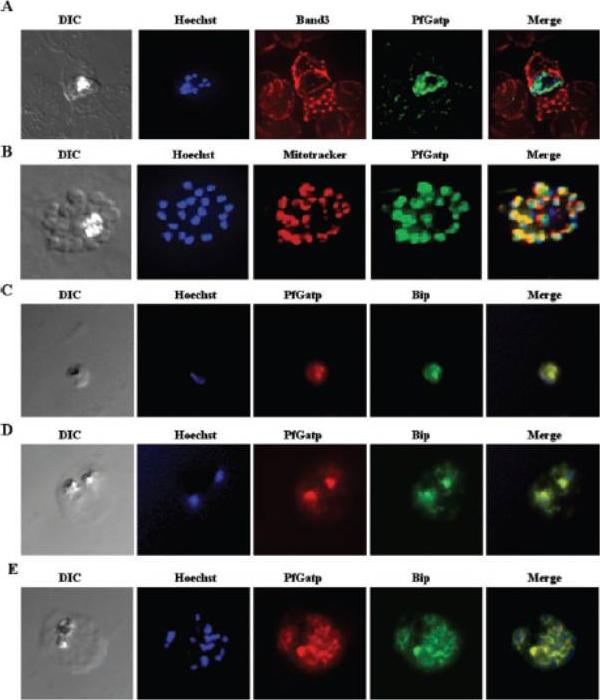
Immunofluorescence microscopy of P. falciparum-infected red blood cells using PfGatp antibodies. A, double-labeling schizont with PfGatp- and Band 3-specific antibodies. In green, PfGatp conjugated to the FITC-conjugated goat anti-rabbit secondary antibody. In red, Band 3 conjugated to the Texas Red-conjugated anti-mouse secondary antibody. B, double-labeling with PfGatp-specific antibodies and MitoTracker. DNA was counterstained with Hoechst. C–E, double-labeling at the ring (C), trophozoite (D), and schizont (E) stages with PfGatp- (red) and BiP-specific antibodies (green). Yellow represents regions of overlap between red and green. The PfGatp signal was proximal and only partly overlapping with MitoTracker (B), whereas the signals of PfGatp and BiP completely overlapped in all the three stages (C–E). Collectively, these data suggest that PfGatp is a component of the endoplasmic reticulum. This staining was proximal to the nucleus as revealed by Hoechst labeling.Santiago TC, Zufferey R, Mehra RS, Coleman RA, Mamoun CB. The Plasmodium falciparum PfGatp is an endoplasmic reticulum membrane protein important for the initial step of malarial glycerolipid synthesis. J Biol Chem. 279:9222-32.
See original on MMP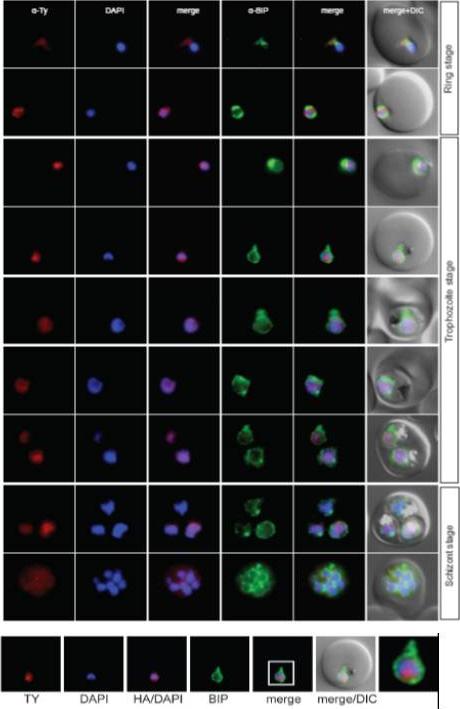
Localisation of PFL0625c during the IDC. Localisation of the tagged protein was visualised using anti-Ty antibodies (red). Antibodies against PfBIP were used to visualise the ER. DAPI was used to visualise the nucleus. DIC images are shown as reference. The bromodomain protein with a more restricted and peripheral localization in ring stages and a diffuse nuclear pattern in trophozoites and schizonts.Oehring SC, Woodcroft BJ, Moes S, Wetzel J, Dietz O, Pulfer A, Dekiwadia C, Maeser P, Flueck C, Witmer K, Brancucci NM, Niederwieser I, Jenoe P, Ralph SA, Voss TS. Organellar proteomics reveals hundreds of novel nuclear proteins in the malaria parasite Plasmodium falciparum. Genome Biol. 2012 Nov 26;13(11):R108.
See original on MMP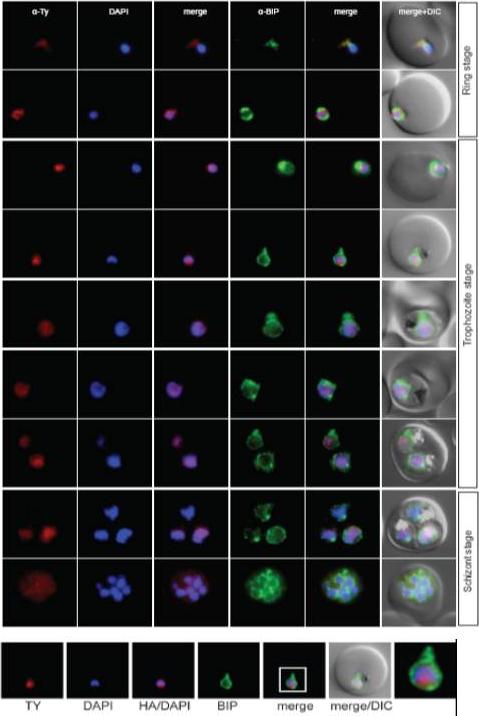
Localisation of PFL0625c during the IDC. Localisation of the tagged protein was visualised using anti-Ty antibodies (red). Antibodies against PfBIP were used to visualise the ER. DAPI was used to visualise the nucleus. DIC images are shown as reference. The bromodomain protein with a more restricted and peripheral localization in ring stages and a diffuse nuclear pattern in trophozoites and schizonts.Oehring SC, Woodcroft BJ, Moes S, Wetzel J, Dietz O, Pulfer A, Dekiwadia C, Maeser P, Flueck C, Witmer K, Brancucci NM, Niederwieser I, Jenoe P, Ralph SA, Voss TS. Organellar proteomics reveals hundreds of novel nuclear proteins in the malaria parasite Plasmodium falciparum. Genome Biol. 2012 Nov 26;13(11):R108.
See original on MMP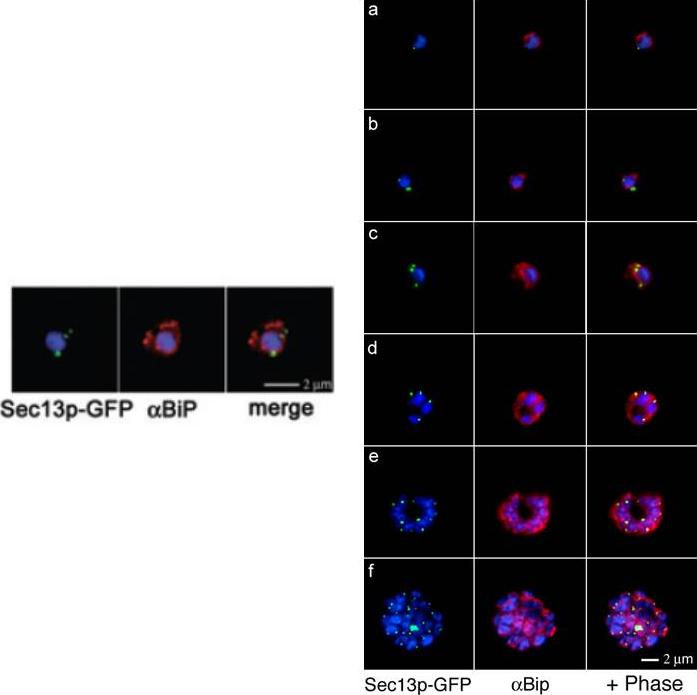
Right: Comparative analyses of transitional ER (tER) dynamics (PfSec13p) with the ER throughout the asexual life cycle of P. falciparum. Transgenic parasites expressing Sec13p–GFP (green) were fixed and stained with antibodies against the ER marker BiP (red). In ring-stage parasites (8-16 hours post invasion) GFP fluorescence is restricted to a tightly defined area in close proximity to the nucleus (a-b). A multiplication of the Sec13p–GFP-defined ER exit site takes place prior to nuclear division (24 hours post invasion, c). As the parasite matures nuclear division commences and the ER forms a more complex network, which is accompanied by further tER site multiplication (d-f).Left: Colocalization of Sec13p–GFP (green) with the ER marker BiP (red) in fixed parasites. Antibodies against the luminal chaperone visualize the ER as a nuclear envelope with some protrusions. Sec13p–GFP is restricted to defined regions within the ER (merged image). All images show the nucleus in blue (DAPI).Struck NS, Herrmann S, Schmuck-Barkmann I, de Souza Dias S, Haase S, Cabrera AL, Treeck M, Bruns C, Langer C, Cowman AF, Marti M, Spielmann T, Gilberger TW. Spatial dissection of the cis- and trans-Golgi compartments in the malaria parasite Plasmodium falciparum. Mol Microbiol. 2008 67:1320-30.
See original on MMP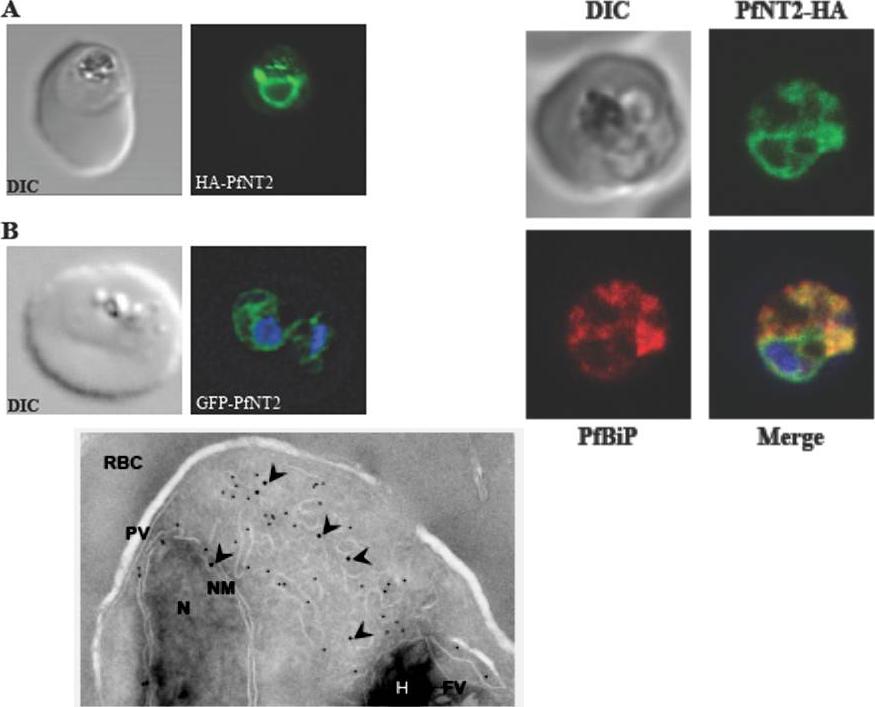
Upper left: Immunolocalization of HA-PfNT2 and GFP-PfNT2. Anti-HA (A) or anti-GFP (B) antibody (green) localizes to a compartment within transgenic parasites expressing either HA-PfNT2 (A) or GFP-PfNT2 (B). The differential contrast image (DIC) images show the location of the parasites within the erythrocyte. The nucleic acids of the GFP-PfNT2 parasites were visualized using Hoescht 33258 (blue). The perinuclear localization observed in these parasites suggested an endoplasmic reticulum location of PfNT2.Upper right: Immunolocalization of carboxy-terminal HA-tagged PfNT2 (PfNT2-HA) to the endoplasmic reticulum. Anti-BiP (red) colocalizes with anti-HA (green). Yellow fluorescence in merged images indicate areas of red (BiP) and green (PfNT2-HA) colocalization. The nucleic acids of the parasite were visualised using Hoescht 33258 (blue).Lower panel: Immunoelectronmicroscopy indicates PfNT2-GFP colocalizes with the ER marker PfBiP. Transmission electron micrograph of ultrathin cryosections of the intraerythrocytic trophozoite stage of P. falciparum PfNT2-GFP transgenic parasites using anti-GFP and anti-PfBiP ntibodies. The image shows immunogold labeling of anti-GFP (18-nm gold particles; indicated with arrow heads) and anti-PfBiP (12-nm gold particles) antibodies bound to intraerythrocytic P. falciparum. N, nucleus; NM, nuclear membrane; PV, parasitophorous vacuole; RBC, red blood cell cytoplasm; FV, food vacuole; H, haemozoin.Downie MJ, El Bissati K, Bobenchik AM, Nic Lochlainn L, Amerik A, Zufferey R, Kirk K, Ben Mamoun C. PfNT2: a permease of the equilibrative nucleoside transporter family in the endoplasmic reticulum of Plasmodium falciparum. J Biol Chem. 2010 285:20827-20833.
See original on MMP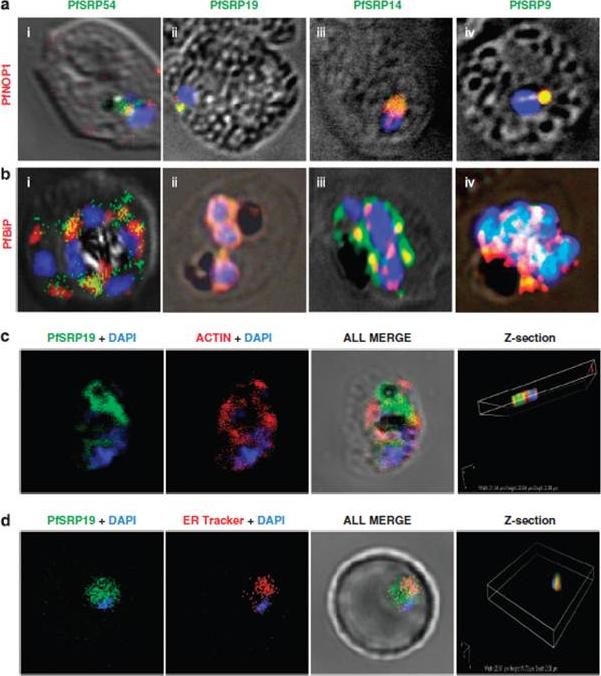
Co-staining of PfSRP polypeptides with nucleolar marker PfNOP1, ER marker PfBiP and cytoplasmic marker Pfactin. (a, b and c) P. falciparum infected erythrocytes stained with anti-PfSRP antibodies (green), anti-PfNOP1 (red), anti-PfBiP (red) and anti-Pfactin (red) respectively. (d) localization of GFP fused SRP19 (green) with ER tracker (red). DAPI is used for nuclear staining (blue). A considerable overlap in staining was observed between three PfSRP polypeptides; PfSRP19, -14, and -9 with PfNOP1 and with PfBiP protein (a and b, i-iv). Importantly, considerable co-localization of PfSRP54 with PfNOP1 in early stages of parasite development i.e. merozoite and ring stages.Panchal M, Rawat K, Kumar G, Kibria KM, Singh S, Kalamuddin M, Mohmmed A, Malhotra P, Tuteja R. Plasmodium falciparum signal recognition particle components and anti-parasitic effect of ivermectin in blocking nucleo-cytoplasmic shuttling of SRP. Cell Death Dis. 2014 Jan 16;5:e994.
See original on MMP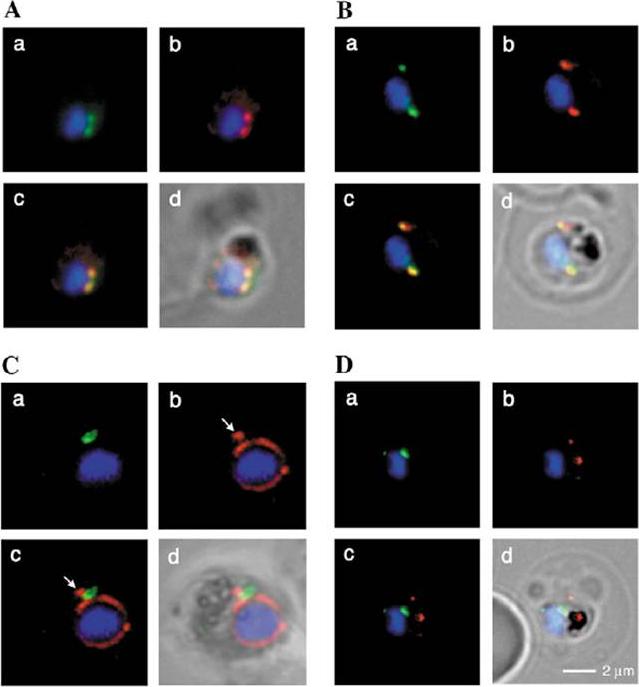
Spatial organisation of the PfGRASP-GFP defined compartment by fluorescence microscopy on fixed parasites. (A) PfGRASP-GFP colocalises with anti PfGRASP-specific antibodies. PfGRASP-GFP is tightly confined to two compartments (a, green) near theparasite nucleus (a, blue). AntiPfGRASP-specificantibodies show a similar staining pattern (b, red withnucleus in blue). Merged image shows the colocalisation of the compartments defined by either PfGRASP-specific antibodies or PfGRASP-GFP expressingparasites (c, yellow). (B) PfGRASP-GFP colocalises with the cis-Golgi marker ERD2. PfGRASP-GFP (a, green) accumulates in two discrete compartments in close proximity to the nucleus (a, blue). Anti-PfERD2 antibodies recognize similar structures (b, red with nucleus in blue). Merged image shows colocalisation of compartments (c, yellow). (C) PfGRASP-GFP defines a compartment that is distinct from the ER . At the early stages of the parasite life cycle (<16 hours post invasion) PfGRASP is restricted to one compartment (a, green) juxtapose to the nucleus (a, blue). The ER is visualised by anti-PfBiP-specific antibodies (b, red). The membranous system of the ER forms an envelope around the nucleus (b, blue) with one protrusion (indicated by arrow). Merged image shows no colocalisation of the two compartments (c). (D) PfGRASP-GFP does not colocalise with the trans-Golgi marker PfRab6. PfGRASP accumulates in two discrete foci (a, green) adjacent to the nucleus (a, blue). Antibodies against PfRab6 visualise two distinct sites within the parasite (b, red with nucleus in blue). Merged image shows no colocalisation of the PfGRASP defined compartment with PfRab6 (c) All panels labelled d in A-D are merges of fluorescent and bright-field images. Bar, 2 mm. Struck NS, de Souza Dias S, Langer C, Marti M, Pearce JA, Cowman AF, Gilberger TW. Re-defining the Golgi complex in Plasmodium falciparum using the novel Golgi marker PfGRASP. J Cell Sci. 2005 118(Pt 23):5603-13.
See original on MMP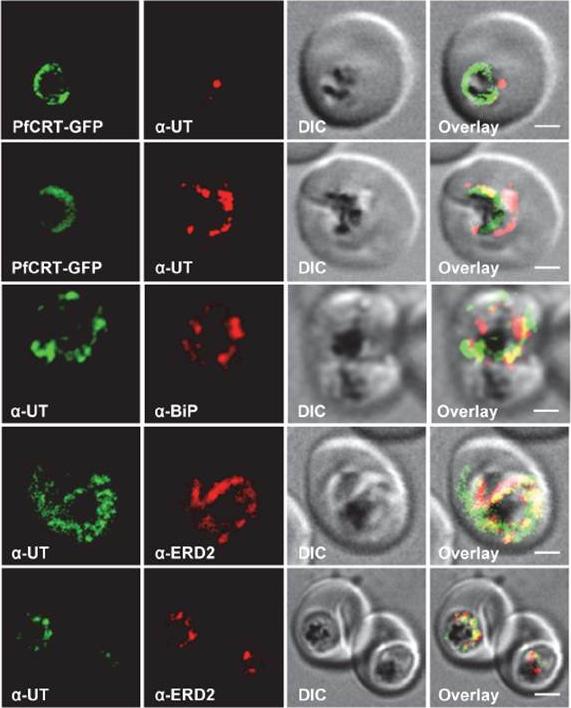
Subcellular localization of PfUT (ubiquitin-transferase). P. falciparum-infected erythrocytes at the trophozoite stage were fixed and analyzed by immunofluorescence assays using antisera to the ER marker BiP (rabbit, 1:1000), the Golgi marker ERD2 (rat, 1:500), and the N- (panels 1 and 5, rabbit, 1:3000; panel 3, mouse, 1:2000) and C-terminal domains of PfUT (panels 2 and 4, rabbit, 1:3000). Panel 1 shows a late ring stage parasite, the other panels show trophozoites. GFP fluorescence was detected, by confocal fluorescence microscopy, in parasites expressing episomally a PfCRT/GFP fusion protein. The different antisera raised against PfUT showed comparable results. Bar, 2 mm. Immunofluorescence microscopy partially co-localized PfUT with the ER marker BiP and the Golgi marker ERD2, but not with PfCRT.Sanchez CP, Liu CH, Mayer S, Nurhasanah A, Cyrklaff M, Mu J, Ferdig MT, Stein WD, Lanzer M. A HECT Ubiquitin-Protein Ligase as a Novel Candidate Gene for Altered Quinine and Quinidine Responses in Plasmodium falciparum. PLoS Genet. 2014 10(5):e1004382.
See original on MMP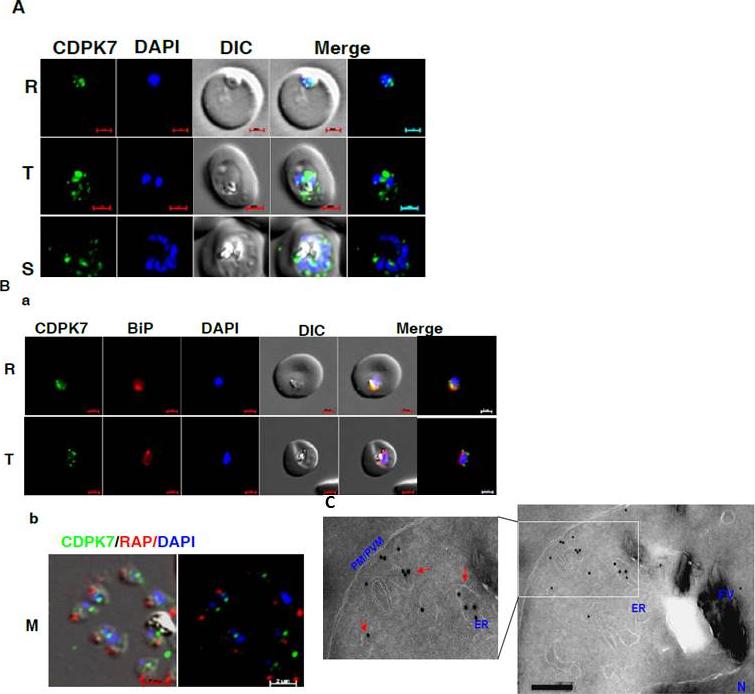
CDPK7 may be present in the ER and vesicles. A IFA was performed to localize CDPK7 in various asexual stages of the parasite using antisera against CDPK7. R, rings; T, trophozoites; S, schizonts. B. Co-staining was done using antisera against CDPK7 and BiP on trophozoites (a) or with anti-RAP1 mAb on free merozoites (b). C. Immuno-EM performed on a trophozoite stage parasite indicated the presence of CDPK7 in ER and vesicular compartments (arrows). Various parasite organelles are indicated: FV, food vacuole; PM/PVM, parasite membrane/parasite vacuolar membrane; ER, Endoplasmic Reticulum; N, Nucleus.Kumar P, Tripathi A, Ranjan R, Halbert J, Gilberger T, Doerig C, Sharma P. Regulation of Plasmodium falciparum development by Calcium-Dependent Protein Kinase 7 (PfCDPK7). J Biol Chem. 2014 Jun 3. [Epub ahead of print]
See original on MMP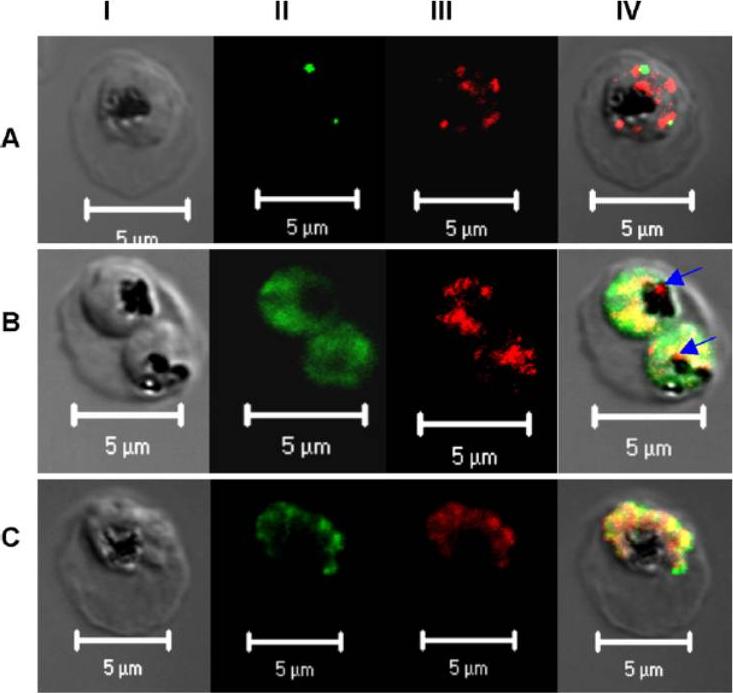
Subcellular localization of PfPRL. Micrographs represent the co-staining profiles of PfPRL with the Golgi marker PfErd2 (A), the ER marker PfBip (B), and the rhoptry/micronemal protein PfAMA-1 (C). Synchronous 3D7 parasite cultures were probed with purified rabbit anti-PfPRL in combination with antibodies to the compartmental markers. Binding of primary antibodies was detected using Alexa-Fluor 488 conjugated antibodies (organellar markers) and Alexa-Fluor 555-conjugated anti-rabbit IgG (PfPRL). Panel I is differential interference contrast image, panel II is fluorescence due to Alexa-Fluor 488 (green), panel III fluorescence due to Alexa-Fluor 555 (red), while panel IV is a merge of the first three panels. Yellow spots indicate overlap between PfPRL and the respective organellar marker, while blue arrows indicate food PfPRL-associated sites in the digestive vacuole.Pendyala PR, Ayong L, Eatrides J, Schreiber M, Pham C, Chakrabarti R, Fidock DA, Allen CM, Chakrabarti D. Characterization of a PRL protein tyrosine phosphatase from Plasmodium falciparum. Mol Biochem Parasitol. 2008 158(1):1-10.
See original on MMP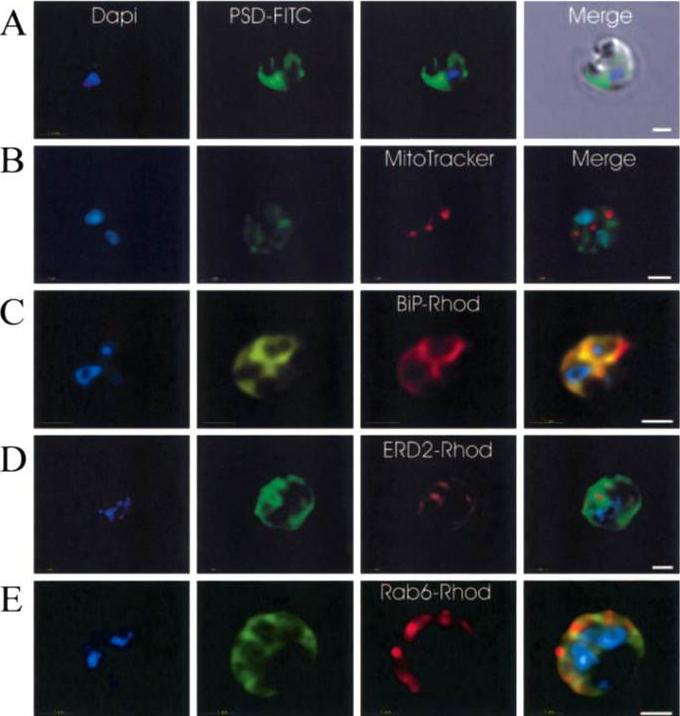
Immunofluorescence localization of PfPSD within P. falciparum-infected erythrocytes. A. FITC (which labels PfPSD) and DAPI (which labels nuclei) images were merged with the Nomarsky image to show the location of PSD labelling in the parasite. DAPI and FITC images were merged with the rhodamine channel corresponding to MitoTracker that labels the mitochondrion (B), BiP (PFI0875w)-rhodamine that labels the ER (C), ERD2 (PF13_0280)-rhodamine that labels the cis-Golgi (D) or Rab6 (PF11_0461)-rhodamine that labels the trans-Golgi (E). All the images except (A) correspond to one selected z-section image after digital deconvolution. The bar corresponds to 1 mm. The entire PfPSD labelling was clearly co-localized with the BiP endoplasmic reticulum marker.Baunaure F, Eldin P, Cathiard AM, Vial H. characterization of a non-mitochondrial type I phosphatidylserine decarboxylase in Plasmodium falciparum. Mol Microbiol. 2004 51:33-46. Copyright John Wiley & Sons Ltd. 2010.
See original on MMP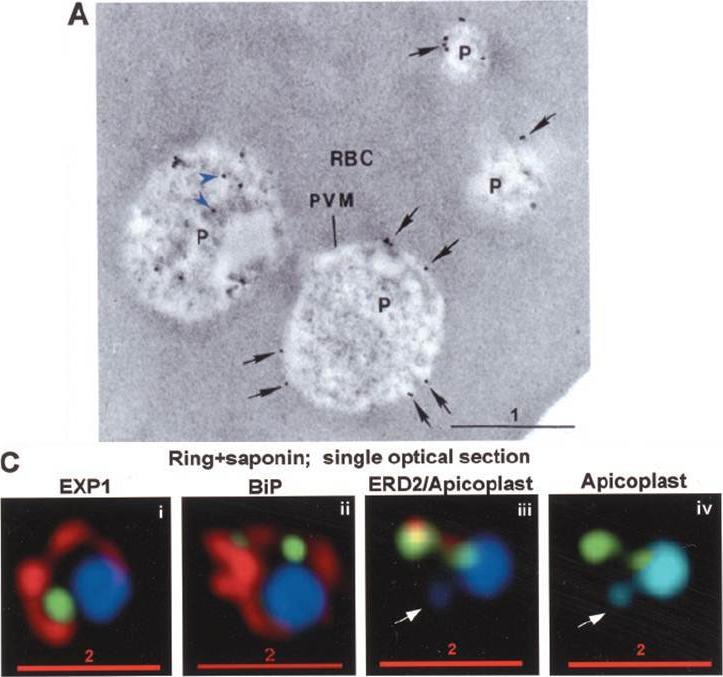
PTS-GFP is detected in the parasitophorous vacuole and in apposition with the Golgi in early rings. A, immunoelectron micrograph of rings showing localization of PTS-GFP in the parasitophorous vacuole and PVM (black arrows) as well as within the parasite (P; bluearrowheads). RBC, red blood cell. Scale bar, 1 mm. Gold particles detecting PTS-GFP are detected at the parasitophorous vacuole and vacuolar membrane (indicated by black arrows) of 6–12-h rings. Internal sites of PTS-GFP staining are also seen. PTS-GFP fluorescence showed some overlap with PfEXP1 a marker for the PVM as well as the Golgi marker PfERD2, consistent with the presence of label in the PV and internal secretory sites. C, i–iv: single optical sections showing green fluorescence in young rings permeabilized with 0.01% saponin relative to secretory markers PfEXP1, PfBiP, PfERD2 (shown in red in i–iii), and apicoplast DNA (marked with an arrow in iii and iv), as detected by indirect immunofluorescence and DeltaVision Microscopy In C, iv, the Hoechst stain is pseudo-colored cyan to facilitate visualization of apicoplast DNA. With 0.01% saponin (, i–iii) revealed loss of the peripheral green fluorescence (C, i–iii). Instead, the saponin-insensitive PTS-GFP associated green fluorescence was largely detectable in a single, major site within the parasite. As expected this site showed no overlap with the PVM marker PfEXP1 (C, i). It also showed no significant overlap with the ER marker BiP (C, ii). PTS-GFP showed no overlap with apicoplast DNA (C, iii and iv; apicoplast DNA is marked with an arrow. However, it was closely apposed to and partially overlapped with the PfERD2 Golgi site (C, iii).Cheresh P, Harrison T, Fujioka H, Haldar K. Targeting the malarial plastid via the parasitophorous vacuole. J Biol Chem. 2002 277(18):16265-77. PMID: 11815606
See original on MMP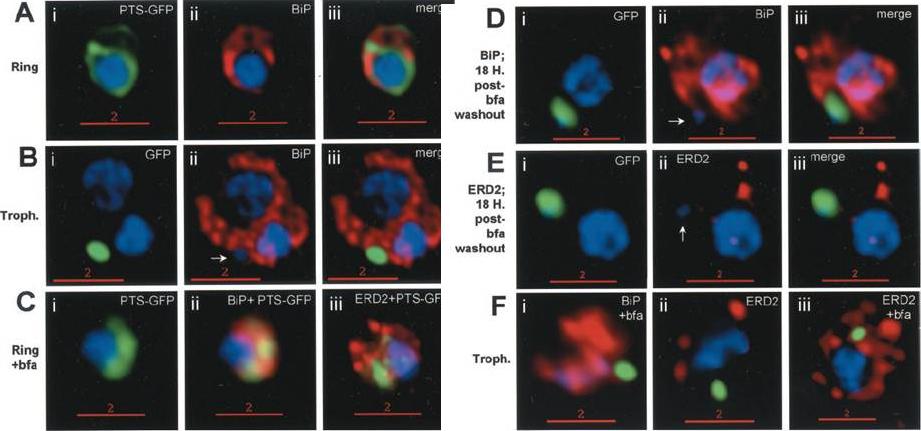
Stage-specific localization of apicoplast-targeted GFP and the effects of brefeldin A (bfa). Distribution of green fluorescence and PfBiP (red) in a early-ring (A, i–iii) and a trophozoite (B, i–iii; a late, ~33 h, trophozoite with twonuclei stained in blue is shown). Distribution of green fluorescence and indicated secretory marker (red) in: rings incubated with Bfa for 24 h (C, i–iii); rings incubated with Bfa for 24 h, washed, and grown for 18 h in absence of drug (D, i–iii; E, i–iii); and trophozoites incubated with or without Bfa (F, i–iii). Blue indicates DNA stained with Hoechst 33342. White arrows in B, ii, D, ii, and E, ii, indicate apicoplast DNA. Scale bars as indicated in microns. PTS-GFP in rings did not show significant overlap with the resident ER marker PfBiP. Nonetheless, its tubular distribution suggested that it resided in one or more membranous compartments. rings were allowed to mature in the presence of Bfa for 24 h (C, i–iii), the distribution of PTS-GFP as well as PfBiP (C, ii) were altered compared with either control rings or trophozoites shown in A and B. In the presence of Bfa, PfBiP lost its reticular staining and PTS-GFP accumulated in diffuse globular regions that show significant overlap with regions of PfBiP stain: however, PTS-GFP and BiP failed to show the identical distribution in Fig. 3C. The Golgi marker PfERD2 was also reorganized by Bfa treatment, consistent with the action of the drug on blocking transport through the Golgi (C, iii).Cheresh P, Harrison T, Fujioka H, Haldar K. Targeting the malarial plastid via the parasitophorous vacuole. J Biol Chem. 2002 277(18):16265-77.
See original on MMP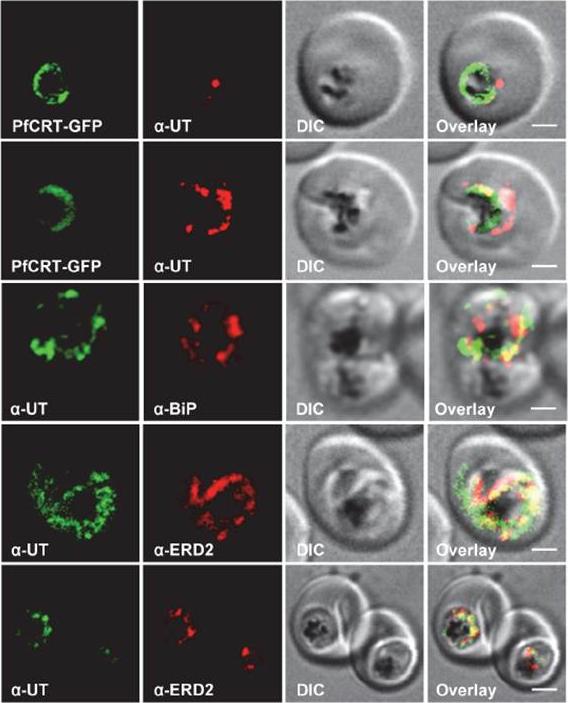
HECT ubiquitin-protein ligase (PfUT) localizes to the ER/Golgi complex. Subcellular localization of PfUT. P. falciparum-infected erythrocytes at the trophozoite stage were fixed and analyzed by immunofluorescence assays using antisera to the ER marker BiP, the Golgi marker ERD2, and the N- (panels 1 and 5, rabbit and C-terminal domains of PfUT. Panel 1 shows a late ring stage parasite, the other panels show trophozoites. GFP fluorescence was detected, by confocal fluorescence microscopy, in parasites expressing episomally a PfCRT/GFP fusion protein. The different antisera raised against PfUT showed comparable results. Bar, 2 mm. Immunofluorescence microscopy partially co-localized PfUT with the ER marker BiP and the Golgi marker ERD2, but not with PfCRT. Quantitative immunoelectron microscopy confirmed a predominant localization of PfUT at the ER/Golgi complex.Sanchez CP, Liu CH, Mayer S, Nurhasanah A, Cyrklaff M, Mu J, Ferdig MT, Stein WD, Lanzer M. A HECT ubiquitin-protein ligase as a novel candidate gene for altered quinine and quinidine responses in Plasmodium falciparum. PLoS Genet. 2014 10(5):e1004382.
See original on MMP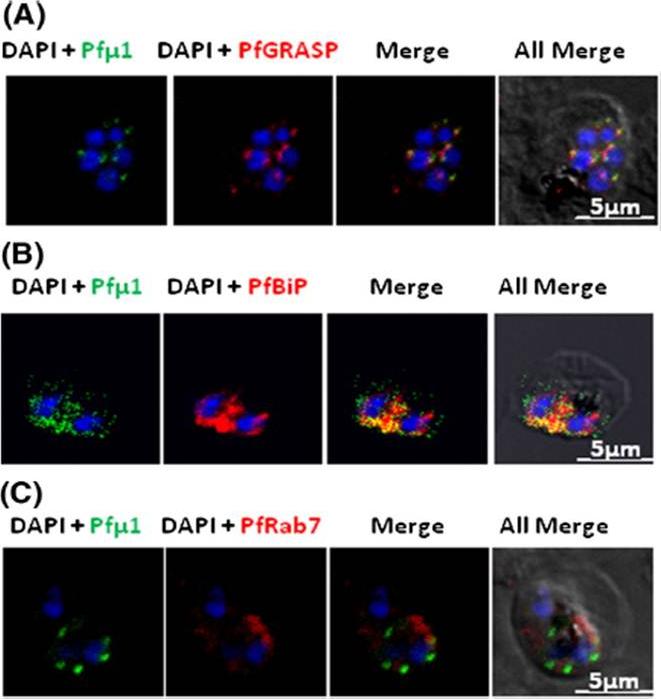
Pfμ1 is closely associated with the Golgi and ER in early trophozoite stages. Transgenic parasites expressing Pfμ1–GFP protein at trophozoite stages were immunostainedwith anti-PfGRASP (A), anti-PfBiP (B) and anti-PfRab7 (C) antibodies. The parasite nuclei were stained with DAPI and slides were visualized by confocal laser scanning microscopy. Scale bars denote 5 μm. Pfμ1 partially co-localized with PfGRASP and PfBip. the ER (which is contiguous with the nuclear envelope) and Golgi in developing merozoites are very closely juxtaposed, and dense vesicular traffic occurs between these two compartments, as well as between nascent rhoptries. No overlap between Pfμ1–GFP and Rab7.This suggests that these proteins do not co-localize, and hence that Rab7 is not involved in trafficking at this stage (3C). These results thus suggest the presence of Pfμ1 in the Golgi–ER network during the early stages of intra-erythrocytic development.Kaderi Kibria KM, Rawat K, Klinger CM, Datta G, Panchal M, Singh S, Iyer GR, Kaur I, Sharma V, Dacks JB, Mohmmed A, Malhotra P. A role for adaptor protein complex 1 in protein targeting to rhoptry organelles in Plasmodium falciparum. Biochim Biophys Acta. 2015 1853(3):699-710.
See original on MMP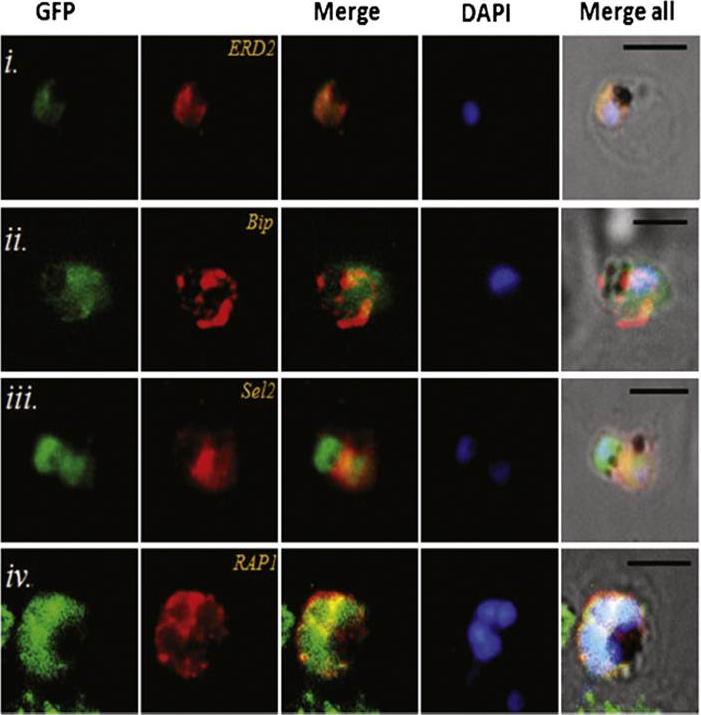
Transgenic parasites expressing Pfμ1–GFP were treated with Brefeldin A (BFA) and immunostained with antibodies specific to cis-Golgi apparatus marker ERD2 (i), endoplasmic reticulum marker, Bip (ii), cytoplasm localized, Sel2 (iii) and RAP1 (iv). The Pfμ1–GFP fusion protein colocalized with Sel2 as well as ERD2 in the parasite cytoplasm upon BFA treatment (i & iii). Parasite nuclei were stained with DAPI; scale bars denote 5 μm. Co-localization with antibodies to ERD2 (a cis-Golgi marker) and BiP, as well as the resident rhoptry protein RAP1 and the cytosolic protein Sel2 showed that Pfμ1 did not show a similar pattern as Bip or Sel2Pfμ1 showed a substantially similar staining pattern as ERD2 which is a Golgi marker (i–iii). Pfμ1 also did co-localize with RAP1 in BFA treated parasites (iv). These observations are consistent with Pfμ1 being Golgi associated at this life stage, consistent with its similar dynamics of redistribution upon brefeldin treatment.Kaderi Kibria KM, Rawat K, Klinger CM, Datta G, Panchal M, Singh S, Iyer GR, Kaur I, Sharma V, Dacks JB, Mohmmed A, Malhotra P. A role for adaptor protein complex 1 in protein targeting to rhoptry organelles in Plasmodium falciparum. Biochim Biophys Acta. 2015 1853(3):699-710.
See original on MMP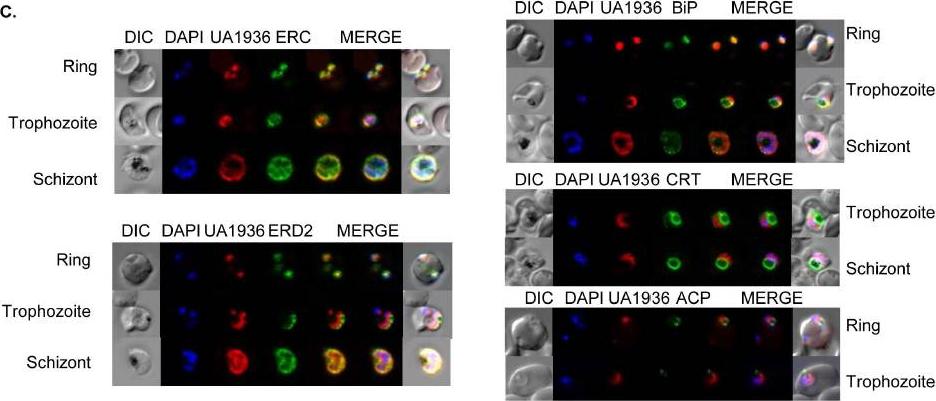
Fluorescence microscopy of UA1936(and inhibitor) and its localization in P. falciparum-infected red blood cells. P. falciparum-infected red blood cells (asynchronous cultures) were cultured with or without 100 mM UA1936 for 1 h and then fixed with 4% paraformaldehyde. In-cell click chemistry was performed with 2.5 mM Alexa 488 alkyne (green) or 2.5 TAMRA alkyne (red) for 30 min. For immunodetection, P. falciparum-infected red blood cells were incubated with specific antibodies against the endoplasmic reticulum markers ERC and BiP, against the cis-Golgi marker ERD2, against the food vacuole membrane marker CRT and against the apicoplast marker ACP.Penarete-Vargas DM, Boisson A, Urbach S, Chantelauze H, Peyrottes S, Fraisse L, Vial HJ. A chemical proteomics approach for the search of pharmacological targets of the antimalarial clinical candidate albitiazolium in Plasmodium falciparum using photocrosslinking and click chemistry. PLoS One. 2014 Dec 3;9(12):e113918. ·
See original on MMP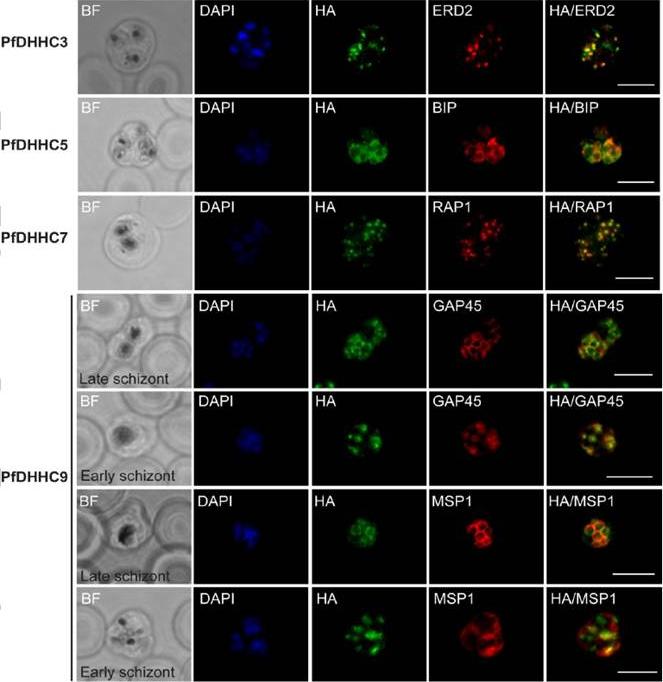
Expression and localisation of PfDHHC proteins in P. falciparum schizonts. Triple-HA-tagged PfDHHC proteins were localised by immunofluorescence using antibodies against the 3-HA tag (green). Immunofluorescence staining of each of the tagged PfDHHC proteins was compared against that of the following known localisation markers (red): ERD2 (Golgi marker), BIP (endoplasmic reticulum marker), RAP1 (rhoptry marker), GAP45 (inner membrane complex marker) and MSP1 (plasma membrane marker). Nuclear staining by DAPI is shown in blue. For the staining of PfDHHC9 with GAP45 and MSP1, both a late schizont, as well as an early schizont, is shown in order to differentiate between IMC and plasma membrane localisation. Scale bar: 5 μm.Tay CL, Jones ML, Hodson N, Theron M, Choudhary JS, Rayner JC. Study of Plasmodium falciparum DHHC palmitoyl-transferases identifies a role for PfDHHC9 in gametocytogenesis. Cell Microbiol. 2016 Apr 6.
See original on MMP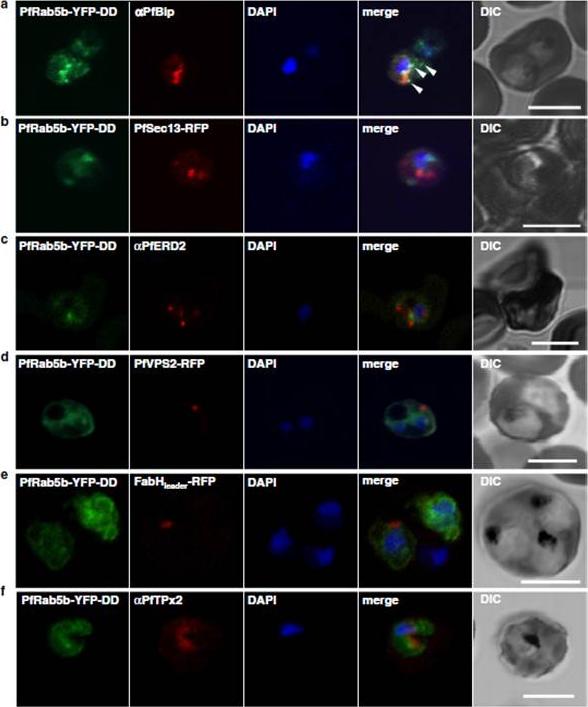
Localization of PfRab5b to a punctate compartment in the parasite cytoplasm. Triple staining with PfRab5b-YFP-DD (green), DAPI (blue) and one of the following markers (red): PfBip (a, ER), PfSec13-RFP (b, ER exit site), PfERD2 (c, Golgi), PfVPS2-RFP (d, putative multivesicular body/endosome), FabHleader-RFP (e, apicoplast), or PfTPx-2 (f, mitochondria) after 24 h incubation with Shld1. PfRab5b-YFP-DD localized adjacent to the Bip signal (arrowheads). Bars 5 μm.Ebine K, Hirai M, Sakaguchi M, Yahata K, Kaneko O, Saito-Nakano Y. Plasmodium Rab5b is secreted to the cytoplasmic face of the tubovesicular network in infected red blood cells together with N-acylated adenylate kinase 2. Malar J. 2016 15:323.
See original on MMP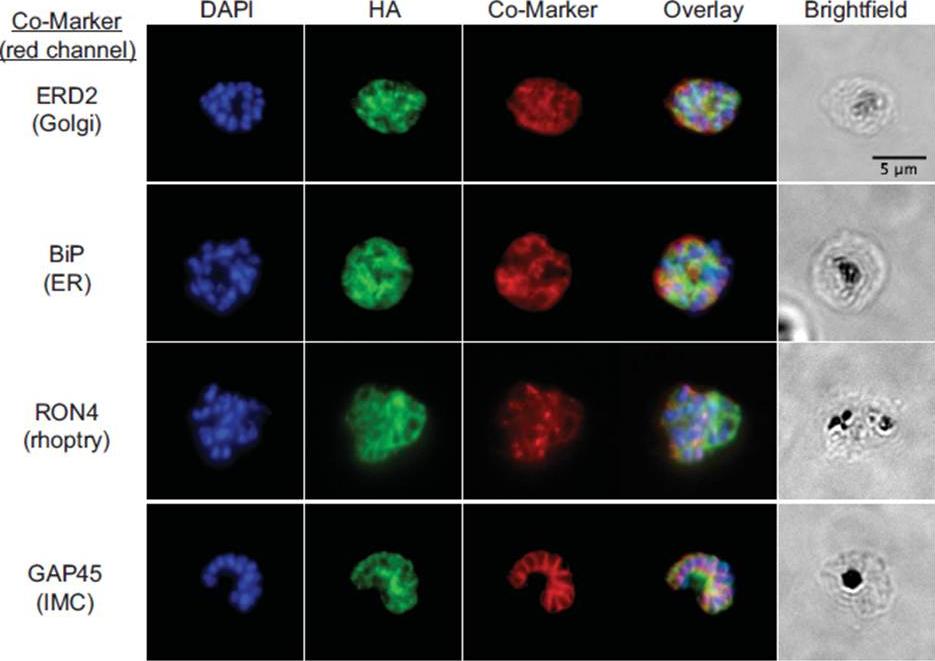
fixed schizont stage parasites were stained with rat α-HA antibody and co-stained with rabbit anti-PfERD2 (1:200), rabbit anti-PfBiP (1:500), mouse anti-PfRON4 (1:100), or rabbit anti-PfGAP45 (1:1000). Primary antibodies were detected with Alexa488-conjugated goat α-rat antibody (1:1000) and Alexa555-conjugated goat α-rabbit or goat anti-mouse (1:1000).Blomqvist K, DiPetrillo C, Streva VA, Pine S, Dvorin JD. Receptor for Activated C-Kinase 1 (PfRACK1) is required for Plasmodium falciparum intra-erythrocytic proliferation. Mol Biochem Parasitol. 2016 Oct 9. pii: S0166-6851(16)30129-3.
See original on MMP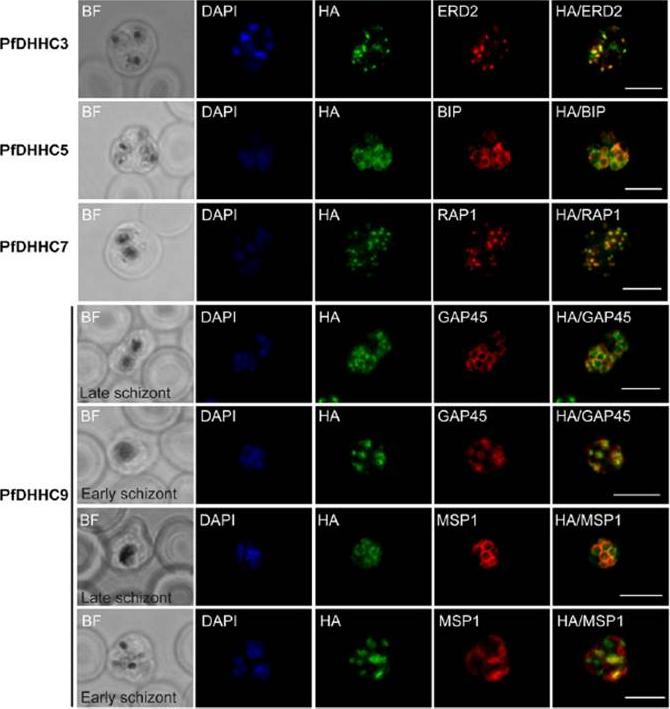
Expression and localization of PfDHHC proteins in Plasmodium falciparum schizonts. Triple-HA-tagged PfDHHC proteins were localized by immuno-fluorescence using antibodies against the 3-HA tag (green). Immunofluorescence staining of each of the tagged PfDHHC proteins was compared against that of the following known localization markers (red): ERD2 (Golgi marker), BIP (endoplasmic reticulum marker), RAP1 (rhoptry marker), GAP45 (inner membrane complex marker) and MSP1 (plasma membrane marker). Nuclear staining by DAPI is shown in blue. For the staining of PfDHHC9 with GAP45 and MSP1, a late schizont, as well as an early schizont, is shown in order to differentiate between inner membrane complex and plasma membrane localization. Scale bar: 5 μm.Tay CL, Jones ML, Hodson N, Theron M, Choudhary JS, Rayner JC. Study of Plasmodium falciparum DHHC palmitoyl transferases identifies a role for PfDHHC9 in gametocytogenesis. Cell Microbiol. 2016 18(11):1596-1610.
See original on MMP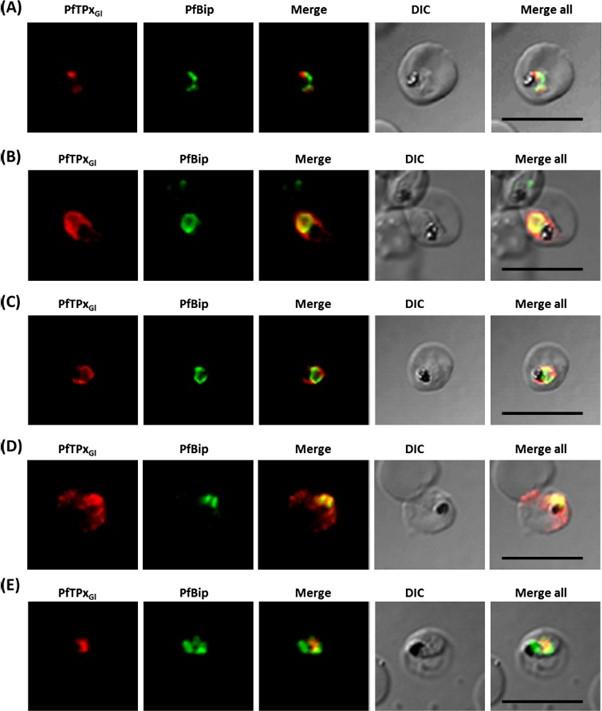
Immunofluorescence images showing the endoplasmic reticulum (ER) morphology in AlF4 and vinblastine treated parasites. (A) PfBiP localization in control parasites for AlF4- treatment, (B) ER morphology in AlF4- treated parasites (14 parasites were counted, none showed dispersal of ER struc-ture), (C) PfBiP localization in solvent (PBS) control parasites for vinblastine treatment, (D) ER morphology in vinblastine-treated parasites (26 parasites were counted, none showed dispersal), (E) ER morphology in parasites reverted after vinblastine treatment (27 parasites counted). Scale Bar: 10 mm. Unlike mammalian cells, no disintegration of the ER was observed in parasites subjected to vinblastine and AlF4-.Both control parasites and drug-treated parasites showed perinuclear ER morphology consistent with the normal development of the ER.Chaudhari R, Dey V, Narayan A, Sharma S, Patankar S. Membrane and luminal proteins reach the apicoplast by different trafficking pathways in the malaria parasite Plasmodium falciparum. PeerJ. 2017 Apr 27;5:e3128.
See original on MMPMore information
| PlasmoDB | PF3D7_0917900 |
| GeneDB | PF3D7_0917900 |
| Malaria Metabolic Pathways | Localisation images Pathways mapped to |
| Previous ID(s) | PFI0875w |
| Orthologs | PBANKA_0818900 , PCHAS_0819200 , PKNH_0715900 , PVP01_0716300 , PVX_099315 , PY17X_0822200 |
| Google Scholar | Search for all mentions of this gene |