PF3D7_0831700 heat shock protein 70 (HSP70-x)
Disruptability [+]
| Species | Disruptability | Reference | Submitter |
|---|---|---|---|
| P. falciparum 3D7 |
Possible |
http://biorxiv.org/content/early/2017/03/03/113365.1 | Theo Sanderson, Wellcome Trust Sanger Institute |
| P. falciparum 3D7 |
Possible |
USF piggyBac screen (Insert. mut.) | USF PiggyBac Screen |
| P. falciparum 3D7 |
Possible |
28732045 ' Although the growth of Δhsp70-x parasites was unaffected, the export of PfEMP1 cytoadherence proteins was delayed and Δhsp70-x IE had reduced adhesion. The Δhsp70-x IE were also more rigid than wild-type controls indicating changes in the way the parasites modified their host erythrocyte. To investigate the cause of this, transcriptional and translational changes in exported and chaperone proteins were monitored and some changes were observed. ' |
Theo Sanderson, Francis Crick Institute |
Mutant phenotypes [+]
| Species | Stage | Phenotype | Reference | Submitter |
|---|---|---|---|---|
| P. falciparum 3D7 | Asexual |
No difference |
http://biorxiv.org/content/early/2017/03/03/113365.1 | Theo Sanderson, Wellcome Trust Sanger Institute |
| P. falciparum 3D7 | Asexual |
No difference |
http://biorxiv.org/content/early/2017/03/03/113365.1
(Conditional)
Attempted with both destabilisation domain and glmS ribozyme |
Theo Sanderson, Wellcome Trust Sanger Institute |
| P. falciparum 3D7 | Asexual |
Attenuated |
31690116 Reduced tolerance of heat shock. |
Theo Sanderson, Francis Crick Institute |
Imaging data (from Malaria Metabolic Pathways)
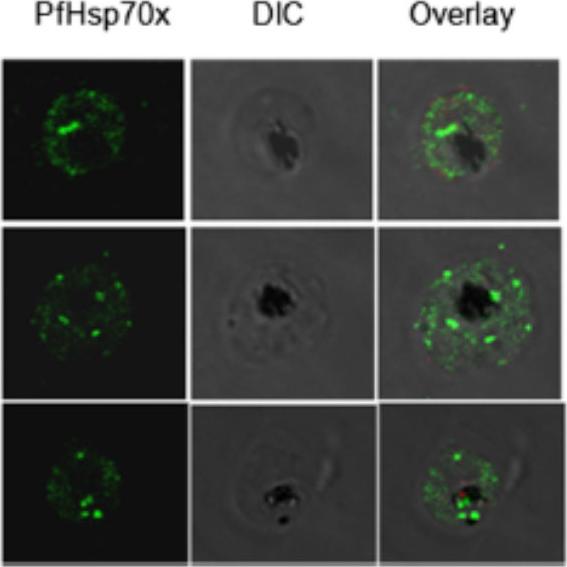
Sub-cellular localization of PfHsp70-x in infected erythrocyte. IFA analysis of infected erythrocytes with PfHsp70-x antiserum reveals its presence throughout the infected erythrocyte. the signal for PfHsp70-x can be seen as punctate spots in the erythrocyte cytosol and around the PVM apart from signal within the parasite. More amount of signal was observed on the inner side of PVM, suggesting that PfHsp70-x gets exported partially.Grover M, Chaubey S, Ranade S, Tatu U. Identification of an exported heat shock protein 70 in Plasmodium falciparum. Parasite. 2013;20:2.
See original on MMP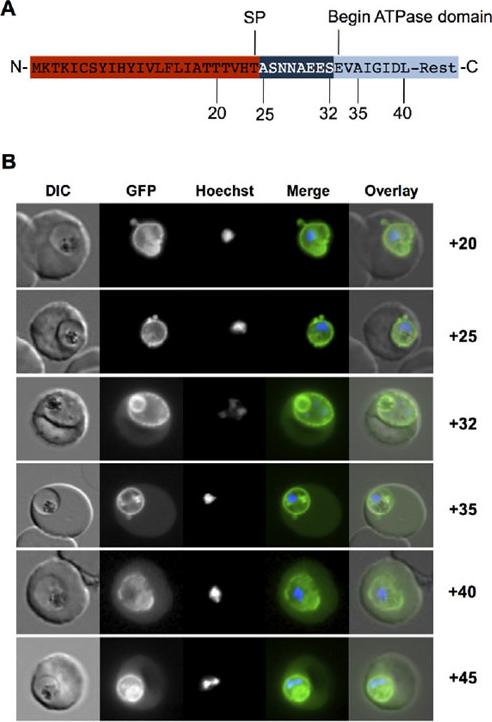
Targeting of PfHsp70-x to the host cell is dependent on an eight-amino-acid sequence. A. Structure of N-terminal region of PfHsp70-x. SP, predicted signal peptide cleavage site. Numbering refers to amino acids from N-terminus. The linker region between putative signal sequence cleavage site and the start of the ATPase domain is shown in dark blue. B. Live cell imaging of parasites expressing various N-terminal GFP fusion chimera. Numbers refer to amino acids from N-terminus. In merge and overlay: green, GFP; blue, Hoechst. Chimera containing up to 25AA show fluorescence exclusively in the PV, whereas chimera containing more than 32AA also show fluorescence in the host cell cytosol.Külzer S, Charnaud S, Dagan T, Riedel J, Mandal P, Pesce ER, Blatch GL, Crabb BS, Gilson PR, Przyborski JM. Plasmodium falciparum-encoded exported hsp70/hsp40 chaperone/co-chaperone complexes within the host erythrocyte. Cell Microbiol. 2012 Aug 1. [Epub ahead of print]
See original on MMP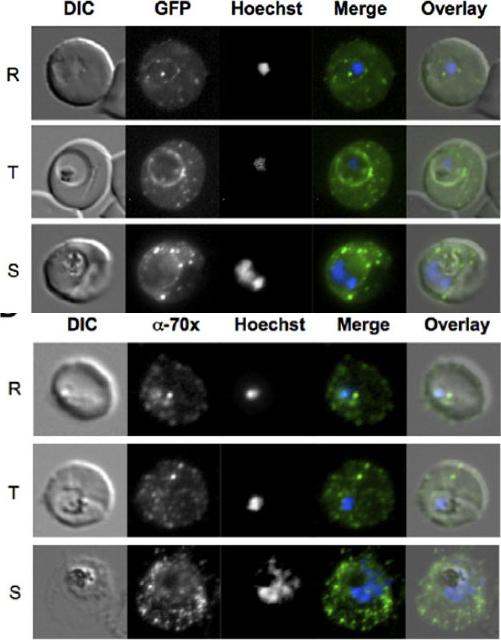
PfHsp70-x localizes to the host cell and PV. Upper panel: Live cell imaging of 3D7G:70x-infected erythrocytes. In the merge and overlay images: green, GFP; blue, Hoechst. In all stages punctate GFP fluorescence can be visualized in the host cell. Additionally, a ring of fluorescence indicative of a PV localization can also be seen. Lower panel: Indirect immunofluorescence localization of PfHsp70-x. In the merge and overlay images: green, a-70x/MRax; blue, Hoechst. R, ring stage; T, trophozoite stage; S, schizont stage.Külzer S, Charnaud S, Dagan T, Riedel J, Mandal P, Pesce ER, Blatch GL, Crabb BS, Gilson PR, Przyborski JM. Plasmodium falciparum-encoded exported hsp70/hsp40 chaperone/co-chaperone complexes within the host erythrocyte. Cell Microbiol. 2012 14(11):1784-95
See original on MMP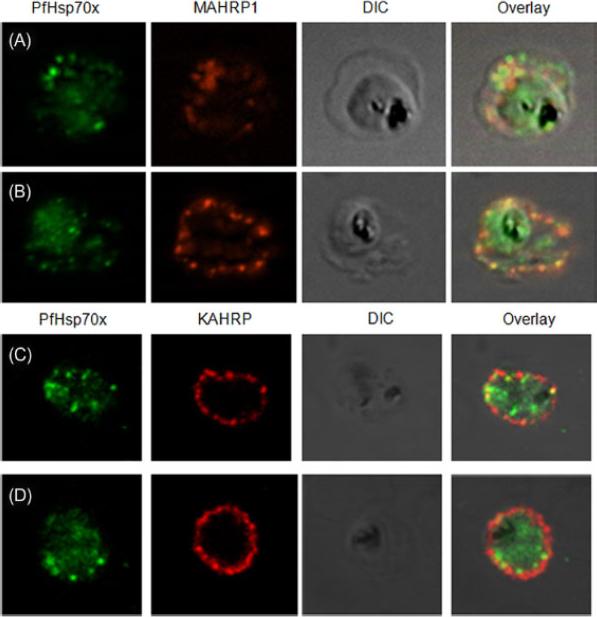
PfHsp70-x partially associates with Maurer’s clefts but not with knobs: (A–B) Panel I: the signal for PfHsp70-x (green) was obtained in the parasite compartment along with few punctate spots in the erythrocyte compartment. Panel II: MAHRP1 (red) stained discrete foci representative of Maurer’s clefts in the erythrocyte periphery. Panel III: the DIC image of the infected erythrocyte. Panel IV: merged image overlaid with DIC image reveals that MAHRP1 and PfHsp70-x partially co-localize in the erythrocyte compartment, suggesting that PfHsp70-x possibly associates with Maurer’s clefts. (C-D) Panel I: the signal for PfHsp70-x (green) was obtained in the parasite compartment along with few punctate spots in the erythrocyte compartment. Panel II: KAHRP (red), being a constituent of knobs, stained the entire erythrocyte membrane. Panel III shows the DIC image of the infected erythrocyte. Panel IV: no co-localization is observed between KAHRP and PfHsp70-x, suggesting that PfHsp70-x does not associate with knobs on the infected erythrocyte membrane.Grover M, Chaubey S, Ranade S, Tatu U. Identification of an exported heat shock protein 70 in Plasmodium falciparum. Parasite. 2013;20:2.
See original on MMP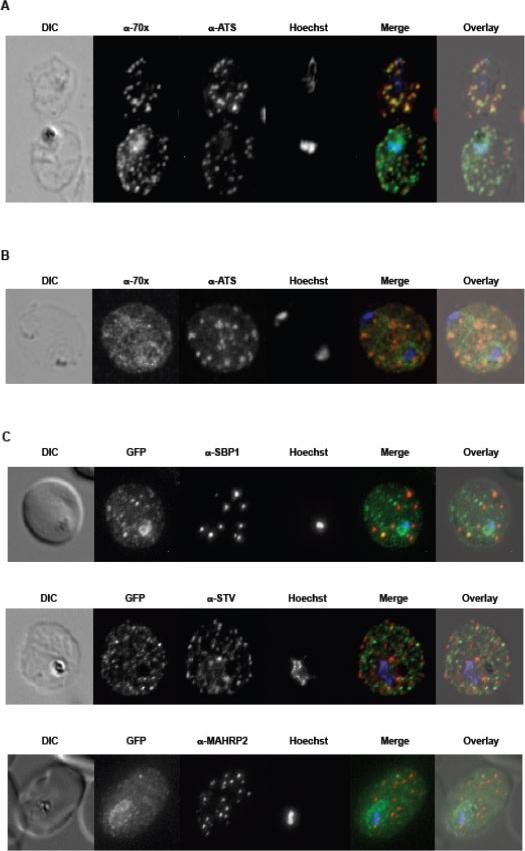
A. tage dependent co-localization of PfHsp70-x and PfEMP1. Upper infected cell (ring stage) shows a higher degree of signal colocalization than lower infected cell (trophozoite). B. ndirect immuno-fluorescence of PfHsp70-x and PfEMP1 using confocal microscopy. In merge and overlay: Green, α-70x; Red, α-ATS; Blue, Hoechst. C. ndirect immuno-fluorescence localization of PfHsp70-x using anti-SBP1, anti-MAHRP2 and anti-STEVOR (STV) antisera in cells infected with 3D7GFP:70x. In merge and overlay images: Green, GFP; red, specific antisera; Blue, Hoechst. No co-localization can be observed between the GFP and antibody-specific signals.Külzer S, Charnaud S, Dagan T, Riedel J, Mandal P, Pesce ER, Blatch GL, Crabb BS, Gilson PR, Przyborski JM. Plasmodium falciparum-encoded exported hsp70/hsp40 chaperone/co-chaperone complexes within the host erythrocyte. Cell Microbiol. 2012 14(11):1784-95
See original on MMP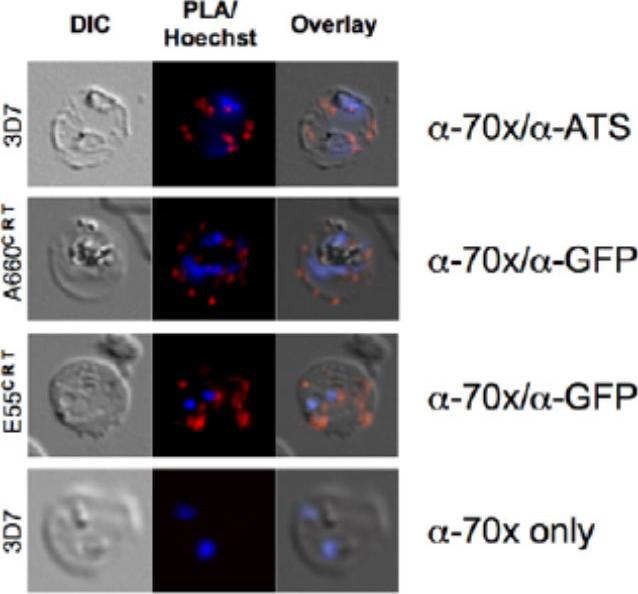
PfHsp70-x interacts with exported Hsp40s and PfEMP1. A. proximity ligation assay (PLA) of (upper panel) PfHsp70-x/PfEMP1, (second panel) PfHsp70-x/A660-GFP (PFA0660w), (third panel) PfHsp70-x/E55-GFP (PFE0055c). A red signal indicates a positive interaction. Lower panel shows negative control. PfHsp70-x appears to closely interact with the ATS of PfEMP1, and both exported Hsp40s studied.Külzer S, Charnaud S, Dagan T, Riedel J, Mandal P, Pesce ER, Blatch GL, Crabb BS, Gilson PR, Przyborski JM. Plasmodium falciparum-encoded exported hsp70/hsp40 chaperone/co-chaperone complexes within the host erythrocyte. Cell Microbiol. 2012 14(11):1784-95
See original on MMP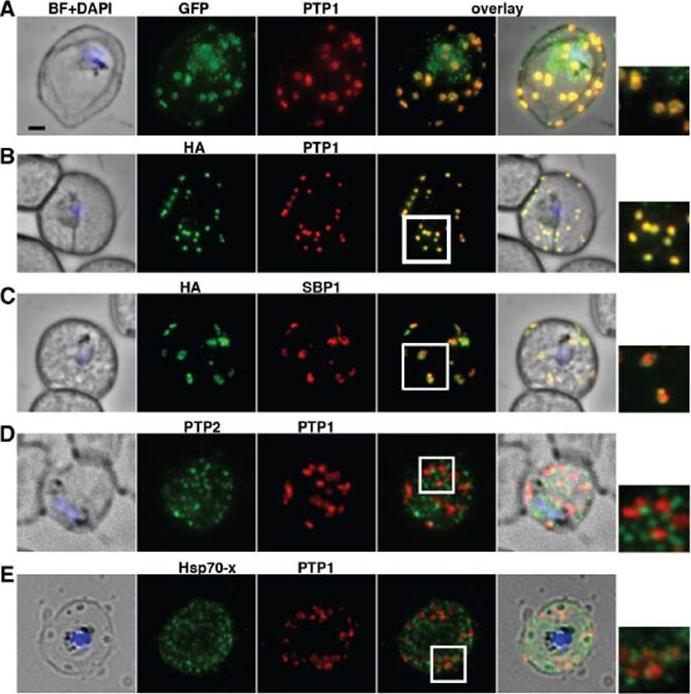
PfPTP1 is localised on Maurer’s clefts in P. falciparum-infected RBCs. (A) Immunofluorescence assay on CS2/PfPTP1-GFP cell line: the GFP signal (green) colocalises with that of the endogenous protein (PfPTP1; red); inset: enlargement of colocalisation pattern. (B) Immuno-fluorescence assay on CS2/PfPTP1-HA cell line: the HA signal (green) colocalises with the α-PfPTP1 signal (red); inset: enlargement of colocalisation pattern. (C) Immunofluorescence assay on CS2/PfPTP1-HA cell line: the HA-signal (green) colocalises partially with the MC resident protein SBP1 (red); inset: enlargement of colocalisation pattern. (D) Immunofluorescence assay on CS2 parental cell line: the PfPTP2-signal (green) does not overlap with the PfPTP1-signal (red); inset: enlargement of area where MC (PfPTP1) and electron dense vesicles (PfPTP2) are in close proximity. (E) Immunofluorescence assay on CS2 parental cell line: the PfHsp70-x-signal (green) colocalises partially with the PfPTP1- signal (red); inset: enlargement of partial colocalisation on J-Dots (PfHsp70-x). Scale bar: 1μm; same size of ROI in each image. Rug M, Cyrklaff M, Mikkonen A, Lemgruber L, Kuelzer S, Sanchez CP, Thompson J, Hanssen E, O'Neill M, Langer C, Lanzer M, Frischknecht F, Maier AG, Cowman AF. Export of virulence proteins by malaria-infected erythrocytes involves remodelling of host actin cytoskeleton. Blood. 2014 Aug 19 [Epub ahead of print]
See original on MMP
GEXP18 and PfPHIST_0801 are J-dot proteins. (A) Immunofluorescence using the J-dot marker PfHsp70x shows good colocalization with the GEXP18GFP chimera. (B) Immunofluorescence using the J-dot marker PfHsp70x shows good colocalization with PfPHIST_0801GFP. 70x, PfHsp70x; DIC, differential interference contrast, PHISTC, PfPHIST_0801. PfHsp70x and PfGEXP18 are found in a common protein complex. As PfGEXP18 is only found at the J-dots (A), this entire complex is likely to be localized to the J-dots. There is a good colocalization between PfPHIST_0801 and PfHsp70x (B) suggesting that PfPHIST_0801, as well as PfGEXP18, localizes to the J-dots.Zhang Q, Ma C, Oberli A, Zinz A, Engels S, Przyborski JM. Proteomic analysis of exported chaperone/co-chaperone complexes of P. falciparum reveals an array of complex protein-protein interactions. Sci Rep. 2017 7:42188.
See original on MMPMore information
| PlasmoDB | PF3D7_0831700 |
| GeneDB | PF3D7_0831700 |
| Malaria Metabolic Pathways | Localisation images Pathways mapped to |
| Previous ID(s) | MAL7P1.228 |
| Orthologs | |
| Google Scholar | Search for all mentions of this gene |