PF3D7_0501300 skeleton-binding protein 1 (SBP1)
Disruptability [+]
| Species | Disruptability | Reference | Submitter |
|---|---|---|---|
| P. falciparum 3D7 |
Possible |
17023587 Absence of PfEMP1 from surface |
Theo Sanderson, Wellcome Trust Sanger Institute |
| P. falciparum 3D7 |
Possible |
USF piggyBac screen (Insert. mut.) | USF PiggyBac Screen |
Mutant phenotypes [+]
None reported yet. Please press the '+' button above to add one.Imaging data (from Malaria Metabolic Pathways)
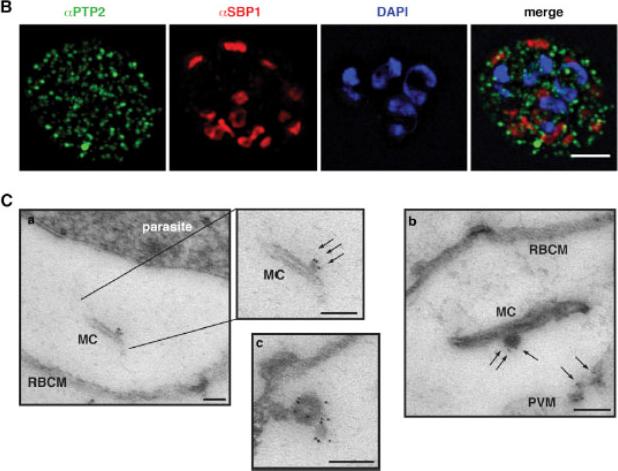
Localization of PfPTP2 in CS2idhfrPTP2/HA-infected RBCs. (B) First panel, PfPTP2 (green); second panel, SBP1 (Maurer’s cleft marker; red); third panel, DAPI (blue); fourth panel, all panels merged. (C) Immuno-EM of CS2idhfrPTP2/HA-infected RBCs after treatment with equinotoxin II. MC, Maurer’s cleft; RBCM, red blood cell plasma membrane; PVM, parasitophorous vacuole membrane. a, the side panel shows higher magnification of Maurer’s cleft; arrows point to budding vesicle where PfPTP2 is localized. b, arrows point to PfPTP2 on budding vesicle and membrane material. c, an example of budding vesicle with PfPTP2 localization. PfPTP2-labeled material in host cell cytoplasm was derived from Maurer’s clefts .Regev-Rudzki N, Wilson DW, Carvalho TG, Sisquella X, Coleman BM, Rug M, Bursac D, Angrisano F, Gee M, Hill AF, Baum J, Cowman AF. Cell-Cell Communication between Malaria-Infected Red Blood Cells via Exosome-like Vesicles. Cell. 2013 May 23;153(5):1120-33.
See original on MMP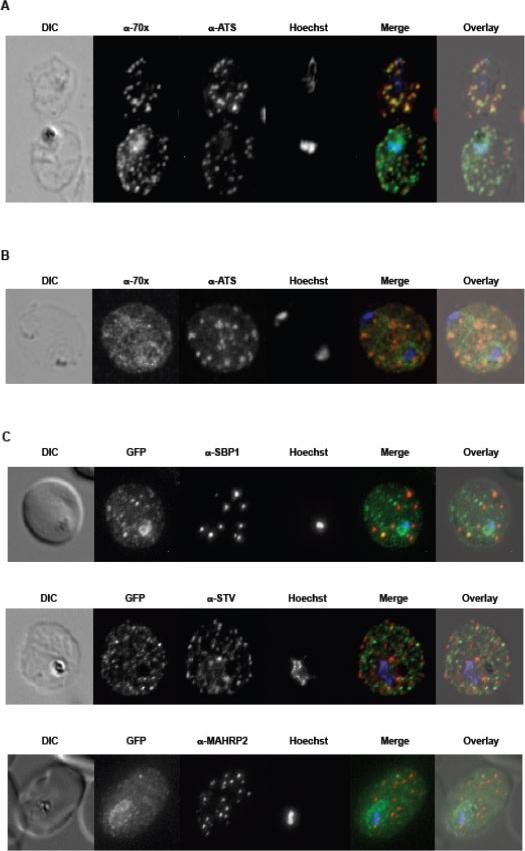
A. tage dependent co-localization of PfHsp70-x and PfEMP1. Upper infected cell (ring stage) shows a higher degree of signal colocalization than lower infected cell (trophozoite). B. ndirect immuno-fluorescence of PfHsp70-x and PfEMP1 using confocal microscopy. In merge and overlay: Green, α-70x; Red, α-ATS; Blue, Hoechst. C. ndirect immuno-fluorescence localization of PfHsp70-x using anti-SBP1, anti-MAHRP2 and anti-STEVOR (STV) antisera in cells infected with 3D7GFP:70x. In merge and overlay images: Green, GFP; red, specific antisera; Blue, Hoechst. No co-localization can be observed between the GFP and antibody-specific signals.Külzer S, Charnaud S, Dagan T, Riedel J, Mandal P, Pesce ER, Blatch GL, Crabb BS, Gilson PR, Przyborski JM. Plasmodium falciparum-encoded exported hsp70/hsp40 chaperone/co-chaperone complexes within the host erythrocyte. Cell Microbiol. 2012 14(11):1784-95
See original on MMP
STEVOR-specific immunofluorescence staining of mature (>24 h after red blood cell invasion) blood-stage P. falciparum D10 (A and C) and representative field isolates K1657 and K1640 from patients in Kilifi, Kenya (B and D), respectively. Parasites were stained using anti-STEVOR peptide 1 antibodies (A and B) or anti-S1 serum (C and D). Early trophozoite (ET), late trophozoite (LT), and schizont (S) stage parasites are shown. All samples were stained with either rabbit anti-STEVOR peptide 1 affinity-purified antibodies (Stevor) or with anti-S1 rabbit serum (STEVOR), as well as the Maurer’s cleft specific anti-PfSBP1 mouse serum (PfSBP1) and the DNA-specific nuclear stain DAPI (at 1 mg/ml). The individual stains and the merged image (Merge) are shown. STEVOR antibody gave a punctate staining pattern throughout the iRBC, present from the late trophozoite stage and throughout early/mid-stage schizont development in 3D7, D10, and K1657 However, in the highly developed segmented schizont, the staining pattern of STEVOR was more diffuse both around the parasite nuclei and throughout the iRBC cytosol, with some potentially associated with the iRBC surface membrane. Blythe JE, Yam XY, Kuss C, Bozdech Z, Holder AA, Marsh K, Langhorne J, Preiser PR. Plasmodium falciparum STEVOR proteins are highly expressed in patient isolates and located in the surface membranes of infected red blood cells and the apical tips of merozoites. Infect Immun. 2008 76(7):3329-36.
See original on MMP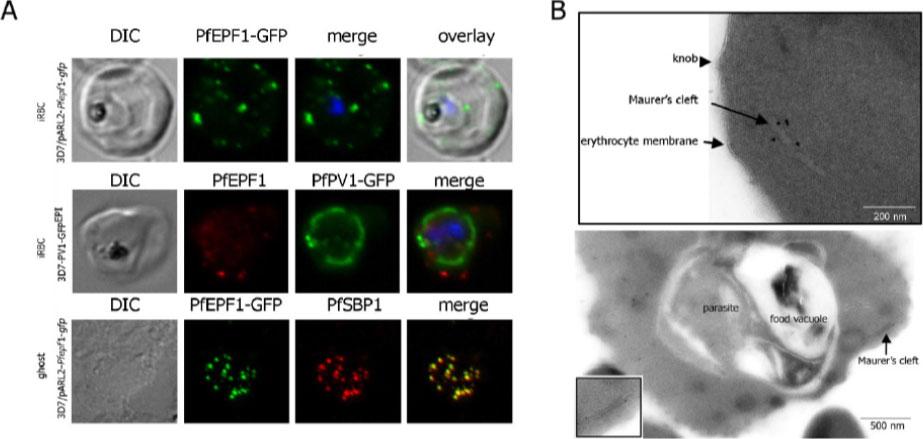
Localization and topology of the PfEPF1 proteins. A. Fluorescent patterns of iRBCs infected by 3D7/pARL2-Pfepf1-gfp (live imaging) and 3D7/PV1-GFPEPI (GFP fluorescence, in green, and immunodetection using anti-PfEPF1 antibodies, in red) and of resealed ghosts from 3D7/pARL2-Pfepf1-gfp iRBCs (GFP fluorescence, in green, and immunodetection using anti-SBP1, in red, antibodies). RBCs and ghost preparations were incubated with DAPI for nucleus labelling. B. Immunoelectron microscopy using anti-GFP antibodies. The inset shows higher magnification of a labelled Maurer’s cleft. Fluorescent dots in the iRBC cytoplasm; some at the erythrocyte periphery are suggestive of Maurer’s clefts while others are observed at the periphery of the parasitophorous vacuole. The Maurer’s cleft localization of the protein was confirmed by its colocalization with PfSBP1.Mbengue A, Audiger N, Vialla E, Dubremetz JF, Braun-Breton C. Novel Plasmodium falciparum Maurer's clefts protein families implicated in the release of infectious merozoites. Mol Microbiol. 2013 88(2):425-42
See original on MMP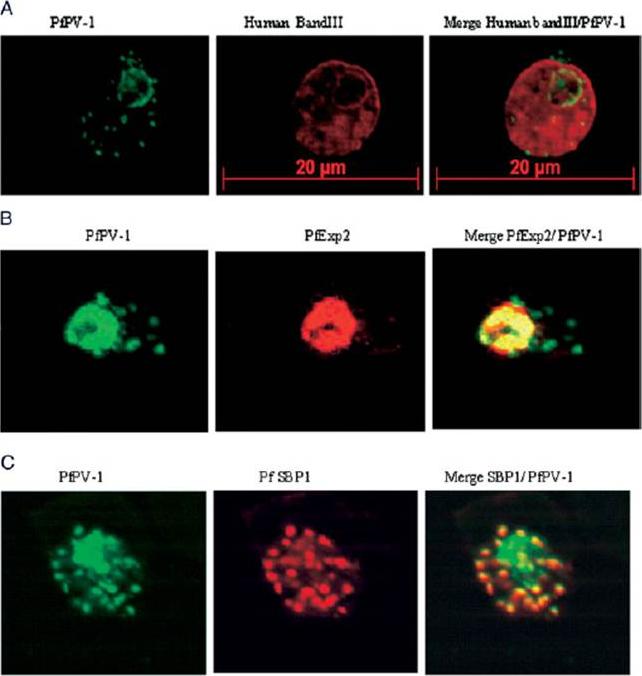
Localization of PfPV-1 in IRBCs. Trophozoite-IRBCs were spread onto microscope slides. Cells were fixed and incubated with mAbs that were directed either against the band 3 protein of the erythrocyte membrane (A), against the vacuolar marker protein PfEXP-2 (B), or against the Maurer’s clefts marker protein PfSBP1 (C). All slides were also incubated with the polyclonal antiserum against PfPV-1. Binding of the mAbs was monitored with Cy3 conjugated anti-mouse secondary antibodies (red), and binding of the polyclonal antiserum was monitored with Cy2 conjugated anti-rabbit secondary antibodies (green). PfPV-1 is concentrated in a distinct ring surrounding the parasite cell, and this location is distinct from that of the erythrocyte membrane (A). It colocalizes with the vacuolar marker protein PfEXP-2 (B) and with PfSBP1 (C), suggesting that it is present not only in the PV but also in Maurer’s clefts.Nyalwidhe J, Lingelbach K. Proteases and chaperones are the most abundant proteins in the parasitophorous vacuole of Plasmodium falciparum-infected erythrocytes. Proteomics. 2006 6:1563-73.
See original on MMP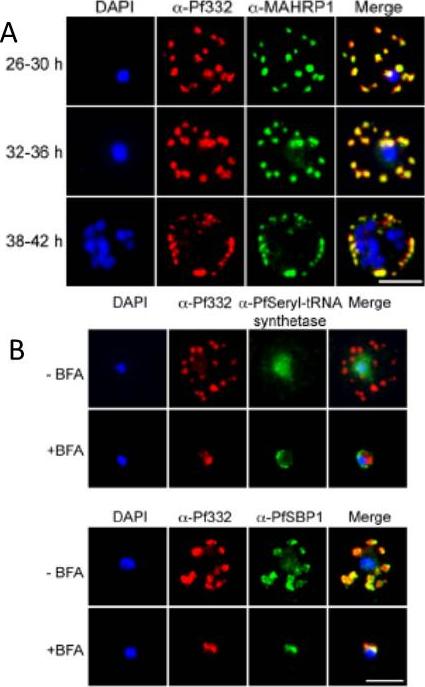
A. IFA of air-dried monolayers of pRBC collected at each of the three indicated time-points. Slides were probed with mouse monoclonal anti-Pf332-DBL (red) and rabbit polyclonal anti-PfMAHRP1 antibodies (green). The parasite was counterstained with DAPI (blue). Scale bar indicates 5 mm. Pf332 displayed a close association with Maurer’s clefts throughout trophozoite maturation and schizogonyB. Air-dried monolayers of BFA+ and BFA2 pRBC were probed with monoclonal anti-Pf332-DBL (red) and polyclonal anti-PfSeryl-tRNA synthetase antibodies (green), or polyclonal anti-Pf332-E200 (red) and polyclonal anti-PfSBP1 antibodies (green). The parasite was counterstained with DAPI (blue). Scale bar indicates 5 mm. Synchronous early ring-stage pRBC were treated with (+) or without BFA for 20 h and inhibition of protein export was verified by IFA. As controls, we included antibodies towards the BFA sensitive SBP1, and the nonexported Seryl-tRNA synthetase. In the absence of BFA, both the anti-Pf332 and anti-SBP1 antibodies stained Maurer’s clefts, whereas the Seryl-tRNA synthetase antibodies only stained the parasiteNilsson S, Angeletti D, Wahlgren M, Chen Q, Moll K. Plasmodium falciparum Antigen 332 Is a Resident Peripheral Membrane Protein of Maurer's Clefts. PLoS One. 2012;7(11):e46980.
See original on MMP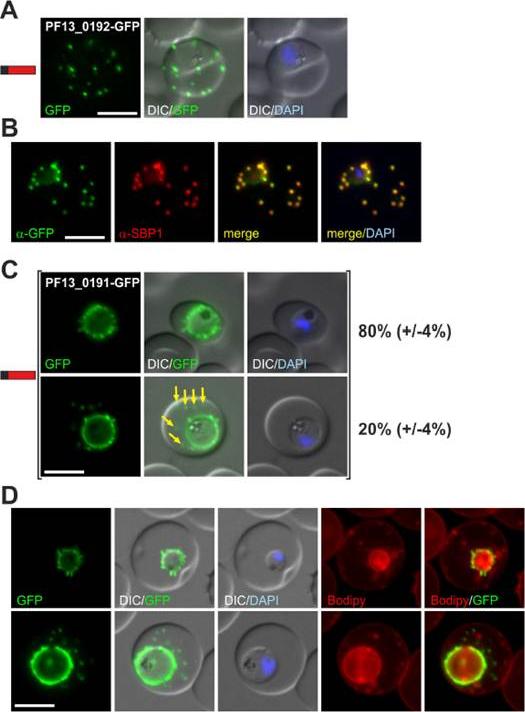
(A) Fluorescence pattern of PF13_0192-GFP. (B) Co-localisation IFA of PF13_0192-GFP with SBP1. (C) Fluorescence pattern of PF13_0191-GFP. Two panels are shown to demonstrate cells with (yellow arrows) and without additional foci of fluorescence in the host cell (ratio indicated in %, at least 50 cells were analysed on 3 occasions, standard deviation in brackets). (D) Bodipy-TR-C5-ceramide (Bodipy) stained parasites expressing PF13_0191-GFP. Protein structure in A and C indicated as in Figure 1. Nuclei were stained with DAPI. Size bars: 5 mm. PF13_0192-GFP was exported to foci in the host cell (A) that were confirmed to be Maurer’s clefts (B). PF13_0191-GFP localized to foci and mobile protrusions at the parasite periphery and in 20% (+/24%) of cells was also found in usually multiple mobile foci in the host cell with no apparent contact to the parasite periphery.Heiber A, Kruse F, Pick C, Grüring C, Flemming S, Oberli A, Schoeler H, Retzlaff S, Mesén-Ramírez P, Hiss JA, Kadekoppala M, Hecht L, Holder AA, Gilberger TW, Spielmann T. Identification of New PNEPs Indicates a Substantial Non-PEXEL Exportome and Underpins Common Features in Plasmodium falciparum Protein Export. PLoS Pathog. 2013 9(8):e1003546.
See original on MMP
Dual label immunofluorescence microscopy of formaldehyde or acetone/methanol-fixed 3D7 smears using antibodies recognizing MAHRP2 (green) and anti-MAHRP1, anti-SBP1 and anti-PfEMP1 (red), or (last panel) anti-MAHRP1 (green) and anti-SBP1 (red). Scale bar = 5 mm. In mature stage parasites the antibodies reacted with punctate structures in the erythrocyte cytoplasm in a pattern that is somewhat reminiscent of Maurer’s cleft staining. No colocalization of MAHRP2 could be found with MAHRP1 MAL13P1.413, SBP1 PFE0065w or PfEMP1; however, the signals were often in close proximity. This suggests that MAHRP2 is associated with a separate compartment of the exomembrane system of P. falciparum.Pachlatko E, Rusch S, Müller A, Hemphill A, Tilley L, Hanssen E, Beck HP. MAHRP2, an exported protein of Plasmodium falciparum, is an essential component of Maurer's cleft tethers. Mol Microbiol. 2010 77(5):1136-52
See original on MMP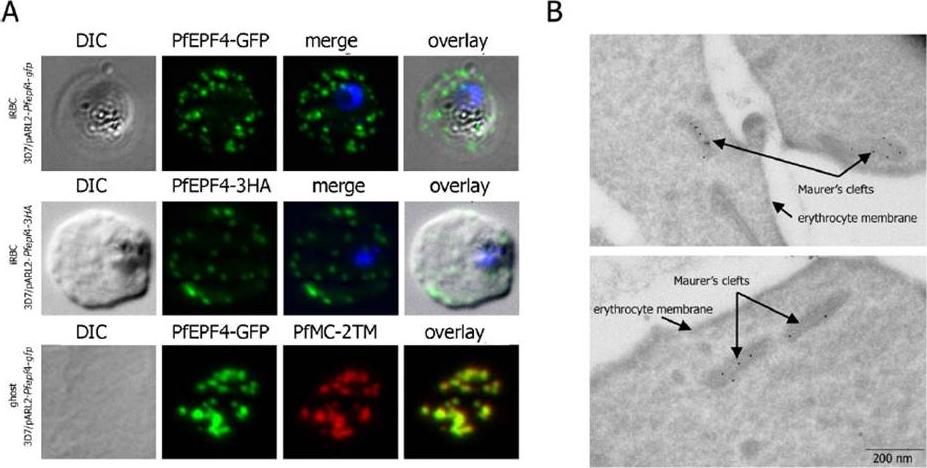
Localization and topology of the PfEPF4 proteins. A. Fluorescent patterns of iRBCs infected by 3D7/pARL2-Pfepf4-gfp (live imaging) and 3D7/pARL2-Pfepf4-3HA (immunodetection using anti-HA antibodies) and of resealed ghosts from 3D7/pARL2-Pfepf4-gfp iRBCs (GFP fluorescence, in green, and immunodetection using anti-PfMC-2TM antibodies, in red). RBCs and ghost preparations were incubated with DAPI for nucleus labelling. B. Immunoelectron microscopy using anti-GFP antibodies. PfEPF4 (A) display a pattern of fluorescent dots in the iRBC cytoplasm. PfEPF4-GFP colocalized with a Maurer’s cleft transmembrane protein, PfSBP1 or PfMC-2TM2 when resealed ghosts from iRBCs were analysed by immunofluorescence. The localization of PfEPF4 at the Maurer’s clefts was confirmed by immunoelectron-microscopy.Mbengue A, Audiger N, Vialla E, Dubremetz JF, Braun-Breton C. Novel Plasmodium falciparum Maurer's clefts protein families implicated in the release of infectious merozoites. Mol Microbiol. 2013 88(2):425-42
See original on MMP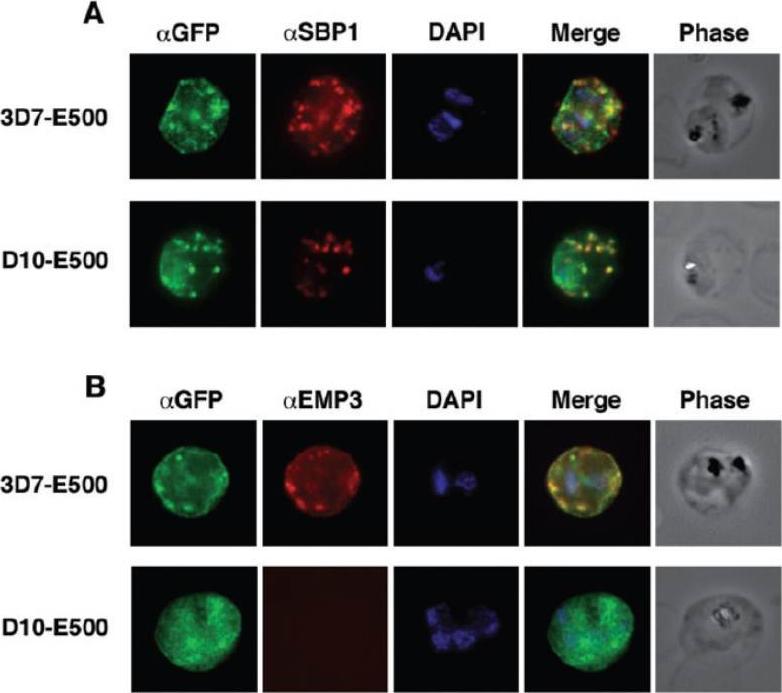
Colocalization of E500-GFP chimeric protein with a Maurer’s clefts marker and native PfEMP3. Indirect mmunofluorescence assays were performed on trophozoite stages of E120-GFP-expressing transgenic 3D7 and E500-GFP-expressing transgenic 3D7 and D10 P. falciparum lines. A. Formaldehyde fixed trophozoite stages were labelled with rabbit αGFP (first column; green), mouse α PfSBP1, a Maurer’s clefts localized protein (second column; red) and DAPI for nuclear staining (third column; blue). The forth column represents the overlay of all three images and the fifth column the phase contrast image of the infected RBC. B. E500-GFP-expressing parasites were fixed and labelled with rabbit αGFP (first column; green), mouse αPfEMP3 (second column; red), an antibody raised against a part of the 3′ repeat region V and DAPI for nuclear staining (third column; blue). The forth column shows the overlays of the three images and the fifth column the phase contrast image of the infected erythrocyte. E120-GFP does not associate with Maurer’s clefts. E500-GFP and PfSBP1, however, colocalize in Maurer’s cleft structures in 3D7-E500. Knuepfer E, Rug M, Klonis N, Tilley L, Cowman AF. Trafficking determinants for PfEMP3 export and assembly under the Plasmodium falciparum-infected red blood cell membrane. Mol Microbiol. 2005 58:1039-53. Erratum in: Mol Microbiol. 2006 59:722
See original on MMP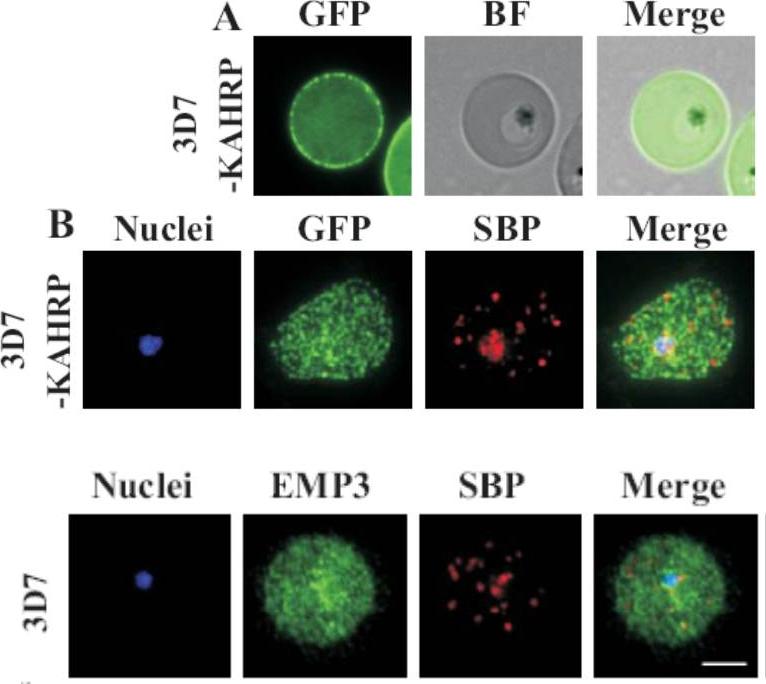
(A) live cell fluorescence microscopy of 3D7-KAHRP-GFP. Panels are GFP fluorescence, bright field (BF) and a merge of the two. Scale bar: 5 μm. KAHRP-GFP was trafficked to the RBC membrane and gave a rim fluorescence pattern (B) Immunofluorescence microscopy of acetone-fixed RBCs infected with 3D7-KAHRP-GFP, SBP1 is a marker for the Maurer’s clefts. Lower row: Immunofluorescence microscopy of acetone fixed RBCs infected with wild type. Smears were probed with mouse anti-PfEMP3 (green) and rabbit anti-SBP1 (red). A characteristic PfEMP3 profile at the RBC membrane is seen.Dixon MW, Kenny S, McMillan PJ, Hanssen E, Trenholme KR, Gardiner DL, Tilley L. Genetic ablation of a Maurer's cleft protein prevents assembly of the Plasmodium falciparum virulence complex. Mol Microbiol. 2011 81(4):982-93.
See original on MMP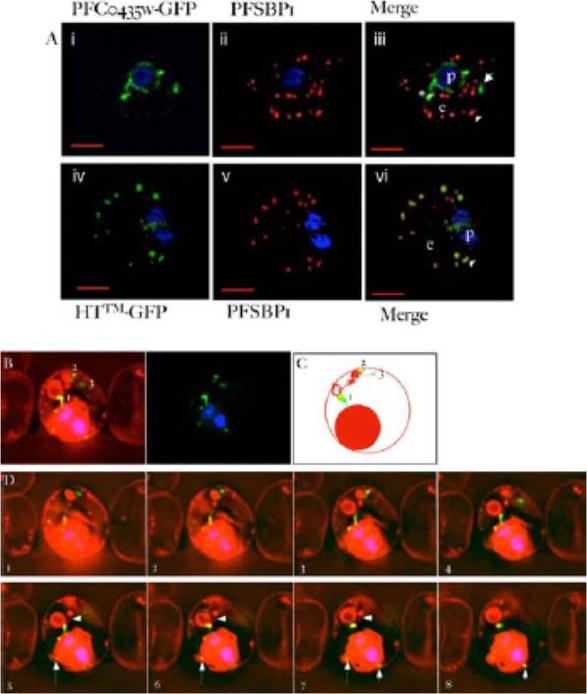
PFC0435w is a junctional protein of the TVN. A) Relative distribution of PFC0435w-GFP (i) or HTTM-GFP (ii) and Maurer’s clefts marked by PfSBP1 (ii, iv) in P. falciparum infected erythrocytes.The cells were also stained with Hoechst 33342 (blue) to visualize the DNA of the parasite. Panels i, ii and iv, v are single optical sections, whose merge is shown in iii and vi respectively. Scale bar, 2 mm. B–D) Association of PFC0435w-GFP with membrane buds and TVN structures B. Zero degree projection of an erythrocyte infected with a PFC0435w- GFP-expressing parasite stained with Rhodamine B. The numbers indicate the large circular TVN structure (1), and PFC0435w-GFP associated with other parts of the TVN (2 and 3). C) Cartoon representation of the infected cell in panel B. D) Optical sectioning of the infected erythrocyte shown in panel B. Distance between sections is 0.2 mm. Note the progression of PFC0435w-GFP staining from the PVM (sections 1–4) to the circular Rhodamine B-stained structure in the erythrocyte (sections 4–8). Arrows indicate PVM buds colocalizing with PFC0435w-GFP. Arrowhead indicates TVN connections between two large loops.van Ooij C, Tamez P, Bhattacharjee S, Hiller NL, Harrison T, Liolios K, Kooij T, Ramesar J, Balu B, Adams J, Waters AP, Janse CJ, Haldar K. The malaria secretome: from algorithms to essential function in blood stage infection. PLoS Pathog. 2008 Jun 13;4(6):e1000084,
See original on MMP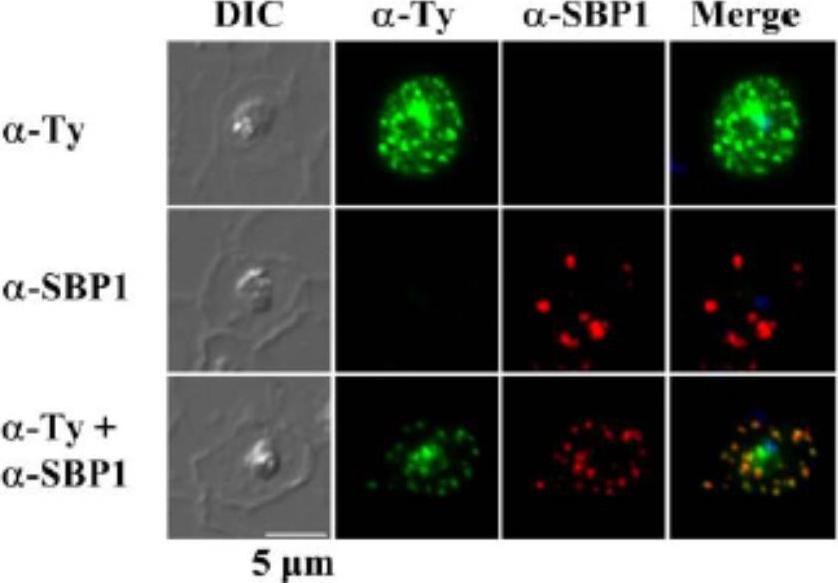
Subcellular localization of rSURFIN4.1 Full (3D7A) in P. falciparum-infected red blood cells. Representative fluorescence images showing the localization of rSURFIN4.1 The differential interference contrast (DIC), fluorescence image with mouse anti-Ty antibody (α-Ty, reacts with Surfin4.1), Maurer’s clefts location with rabbit anti-SBP1 antibody (α-SBP1), and merged image with nucleus staining with DAPI (Merge) are shown. rSURFIN4.1 exhibits a dotted fluorescence pattern within the red blood cell cytosol, co-localized with PfSBP1. Bar = 5 μm.Zhu X, Yahata K, Alexandre JS, Tsuboi T, Kaneko O. The N-terminal segment of Plasmodium falciparum SURFIN(4.1) is required for its trafficking to the red blood cell cytosol through the endoplasmic reticulum. Parasitol Int. 2013 62(2):215-29
See original on MMP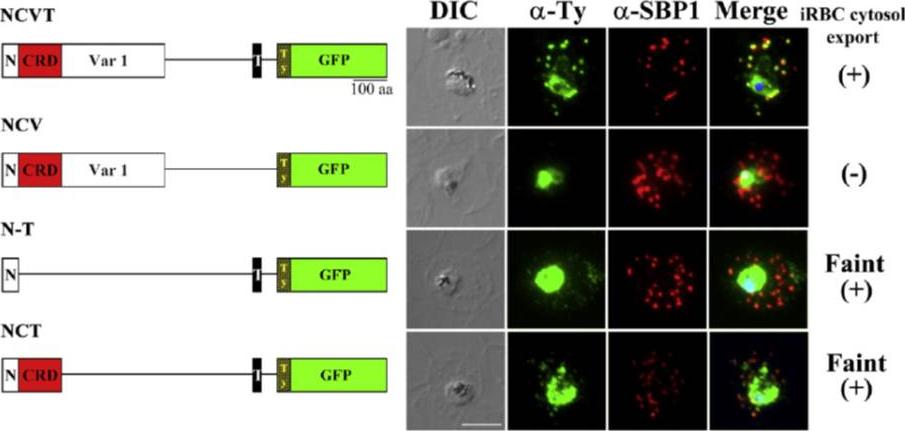
Regions of SURFIN4.1 required for the export to the Maurer's clefts. (A) Schematic drawings of the recombinant proteins, rSURFIN4.1 NCVT (NCVT), rSURFIN4.1 NCV (NCV), rSURFIN4.1 NCT (NCT), and rSURFIN4.1 N-T (N-T) are depicted in the left panels. The right panels show co-localization of a series of recombinant SURFIN4.1 detected with anti-Ty antibody (α-Ty) and counter-staining with PfSBP1 (α-SBP1) by IFA. The differential interference contrast (DIC) and merged images with nucleus stained with DAPI (Merge) are shown. (+) or (−) indicates positive or negative detection of the signals in the iRBC cytosol, respectively. Bar=5 μm. dotted fluorescence signal pattern in the iRBC cytosol, which co-localized with a MC marker PfSBP1. TM region is essential for export from the parasite to the PV lumenZhu X, Yahata K, Alexandre JS, Tsuboi T, Kaneko O. The N-terminal segment of Plasmodium falciparum SURFIN4.1 is required for its trafficking to the red blood cell cytosol through the endoplasmic reticulum. Parasitol Int. 2012 62(2):215-229.
See original on MMP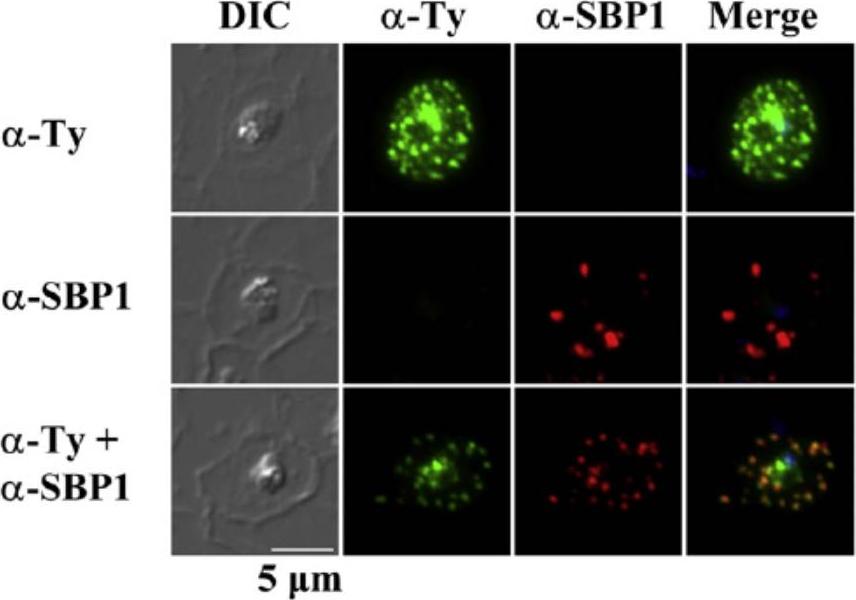
Subcellular localization of rSURFIN4.1Full (3D7A) in P. falciparum-infected red blood cells. Representative fluorescence images showing the localization of rSURFIN4.1Full. The differential interference contrast (DIC), fluorescence image with mouse anti-Ty antibody (α-Ty; recognizing rSURFIN4.1), Maurer's clefts location with rabbit anti-SBP1 antibody (α-SBP1), and merged image with nucleus staining with DAPI (Merge) are shown. rSURFIN4.1Full exhibits a dotted fluorescence pattern within the red blood cell cytosol, co-localized with PfSBP1. Bar=5 μm.Zhu X, Yahata K, Alexandre JS, Tsuboi T, Kaneko O. The N-terminal segment of Plasmodium falciparum SURFIN4.1 is required for its trafficking to the red blood cell cytosol through the endoplasmic reticulum. Parasitol Int. 2012 62(2):215-229.
See original on MMP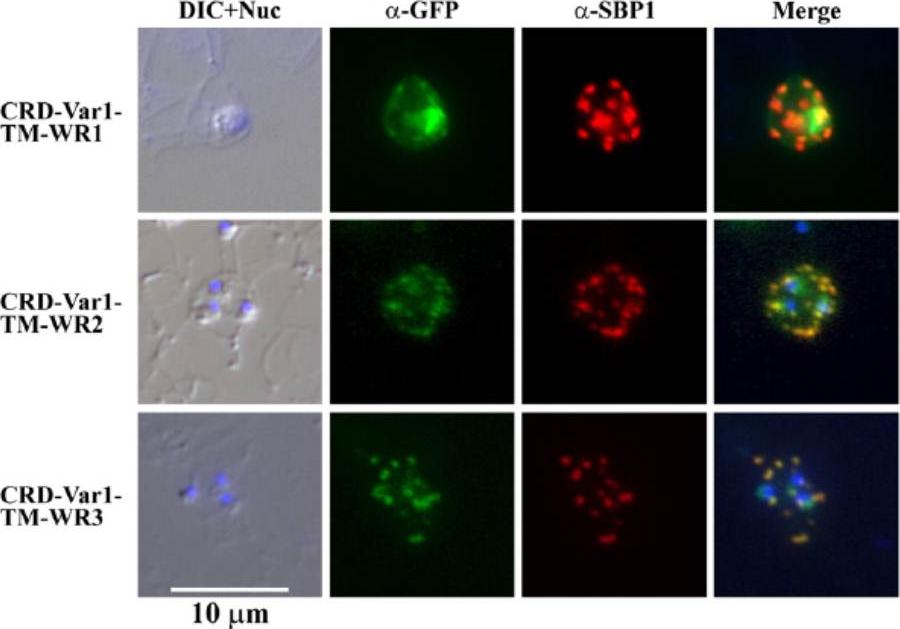
Indirect immunofluorescence assay of three mini-SURFIN4.2 proteins. Double staining IFA for 3 mini-SURFIN4.2-expressing transfectants is shown. α-GFP and α-SBP1 indicate anti-GFP (mini-SURFIN4.2) and anti-SBP1 (Maurer's cleft protein). Negative controls using normal rabbit antibody did not produce detectable signals (not shown). CRD, cysteine-rich domain; TM, transmembrane; and Var1, variable region 1; WR, tryptophan-rich.Alexandre JS, Yahata K, Kawai S, Torii M, Kaneko O. PEXEL-independent trafficking of Plasmodium falciparum SURFIN(4.2) to the parasite-infected red blood cell and Maurer's clefts. Parasitol Int. 2011 60(3):313-20.
See original on MMP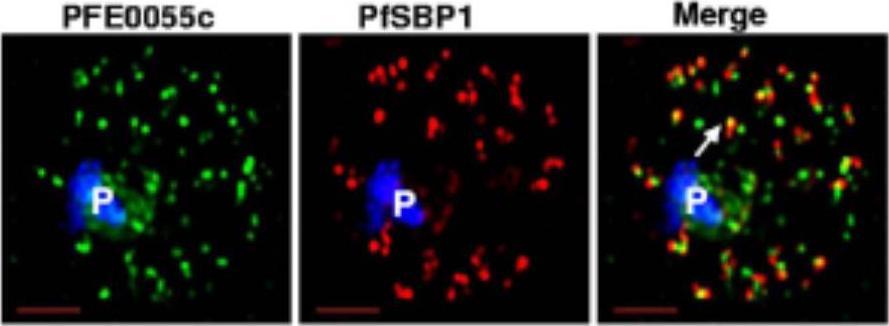
Single optical section of a trophozoite-infected erythrocyte fixed and probed with peptide antibodies to PFE0055c (green) and the cleft protein SBP1 (red). Arrow in merge image shows proximal location of PFE0055c to clefts.Bhattacharjee S, van Ooij C, Balu B, Adams JH, Haldar K. Maurer's clefts of Plasmodium falciparum are secretory organelles that concentrate virulence protein reporters for delivery to the host erythrocyte. Blood. 2008 111:2418-26.
See original on MMP
Single optical section of a trophozoite-infected erythrocyte fixed and probed with peptide antibodies to PFE0055c (green) and the cleft protein SBP1 (red). Arrow in merge image shows proximal location of PFE0055c to clefts.Bhattacharjee S, van Ooij C, Balu B, Adams JH, Haldar K. Maurer's clefts of Plasmodium falciparum are secretory organelles that concentrate virulence protein reporters for delivery to the host erythrocyte. Blood. 2008 111:2418-26.
See original on MMP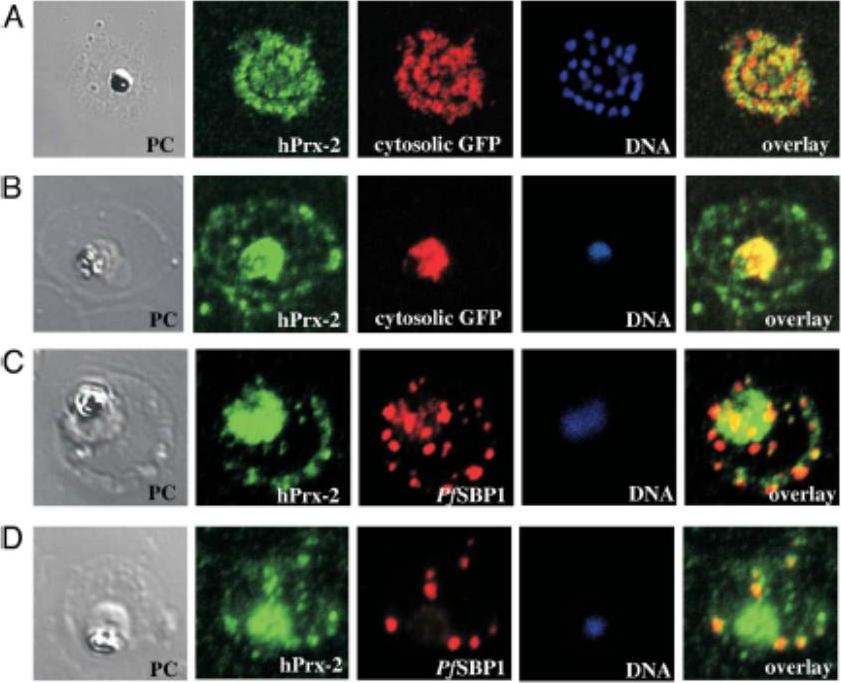
Confocal laser scanning IFA colocalization analyses of hPrx-2 in infected RBCs. (A) IFA colocalization of hPrx-2 (human peroxiredoxin 2) with cytosolic GFP in P. falciparum late schizont/ merozoites. The surrounding RBC is lysed and merozoites are about to be released. Phase contrast (PC) shows the residual hemozoin crystal and merozoites. DNA staining (blue) visualizes 28 merozoites. Cytosolic GFP (red) stains the merozoites and anti-hPrx-2-staining shows presence of hPrx-2 in merozoites (hPrx-2, green). ‘‘Overlay’’ shows a weak colocalization of GFP and hPrx-2. (B) IFA colocalization analysis of hPrx-2 in a trophozoite. PC shows the parasite and the FV containing hemozoin. Anti-hPrx-2-staining (green) reveals the presence of the protein in the trophozoite cytosol as it colocalizes with cytosolic GFP (red). (C and D) IFA colocalization of hPrx-2 with PfSBP1 in P. falciparum trophozoites. PfSBP1 (red) stains the MCs of the trophozoite. Anti-hPrx-2-staining shows presence of hPrx-2 in parasite cytosol and focal staining in the RBC (hPrx-2, green). ‘‘Overlay’’ shows colocalization of hPrx-2 and PfSBP1 in the Maurer’s Clefts.Koncarevic S, Rohrbach P, Deponte M, Krohne G, Prieto JH, Yates J 3rd, Rahlfs S, Becker K. The malarial parasite Plasmodium falciparum imports the human protein peroxiredoxin 2 for peroxide detoxification. Proc Natl Acad Sci U S A.2009 106:13323-8. PMID:
See original on MMP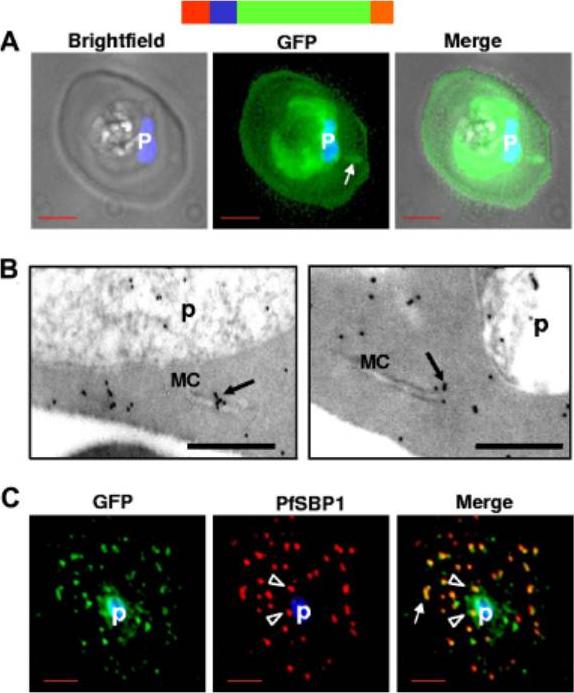
HTsol-GFP, a minimal soluble reporter exported to the erythrocyte cytoplasm and detected in clefts. (A) 0° projection of an erythrocyte infected with transgenic parasites expressing HTsol-GFP. Arrow indicates GFP-labeled intraerythrocytic structure, possibly a cleft. (B) Immunoelectron micrographs of trophozoite parasite (p)-infected cells expressing HTsol-GFP. Ultrathin sections were probed with antibodies to GFP and secondary antibody gold (10 nm) conjugate. Arrows indicate gold particles at intraerythrocytic Maurer’s clefts (MC). No gold labeling was detected in absence of primary antibody or when a nonspecific primary was used (not shown). Bar, 200 nm. (C) Single optical section of an infected erythrocyte expressing HTsol-GFP. Samples were treated to release soluble GFP, fixed, and probed with antibodies to GFP (green) and P falciparum Skeletal Binding Protein1 (PfSBP1, red). Arrow, GFP labeled cleft structures at the periphery of infected erythrocyte; arrowheads, clefts proximal to the parasite. In fluorescence micrographs, p denotes parasite nucleus stained with Hoechst 33342; bar, 2 mm. Schematic representation of the construct is indicated above with ER-type signal sequence (red), sequence containing HT signal (blue) fused to GFP (green) and myc (orange).Bhattacharjee S, van Ooij C, Balu B, Adams JH, Haldar K. Maurer's clefts of Plasmodium falciparum are secretory organelles that concentrate virulence protein reporters for delivery to the host erythrocyte. Blood. 2008 111:2418-26.
See original on MMP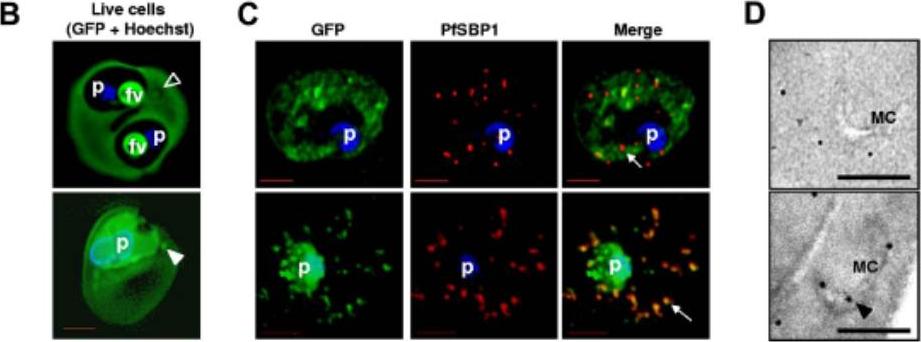
Lumenal association of HTsol-GFP at Maurer’s clefts. (B) 0° projections of an rHT-GFP–loaded erythrocyte ghost infected with 3D7 P falciparum (top) or a mock-loaded erythrocyte ghost infected with transgenic parasite expressing HTsol-GFP (bottom). Empty arrowhead, cleft structure not labeled with intraerythrocytic rHT-GFP; solid arrowhead, GFP labeled cleft. rHT-GFP was found to be uniformly distributed in the infected erythrocyte cytoplasm as well as the food vacuole of parasite (presumably via cytostome-mediated uptake of host cytoplasm). (C) Cells in panel B fixed, permeabilized, and probed with antibodies to GFP (green) and resident cleft protein PfSBP1 (red). Arrows show clefts. (D) Immunoelectron microscopy of cells in panel B showing distribution of GFP associated with Maurer’s clefts (MC). Bar indicates 500 nm. When resealed ghosts were infected with parasites that biosynthetically expressed secretory HTsol-GFP, green fluorescence was quantitatively associated with the clefts .Bhattacharjee S, van Ooij C, Balu B, Adams JH, Haldar K. Maurer's clefts of Plasmodium falciparum are secretory organelles that concentrate virulence protein reporters for delivery to the host erythrocyte. Blood. 2008 111:2418-26.
See original on MMP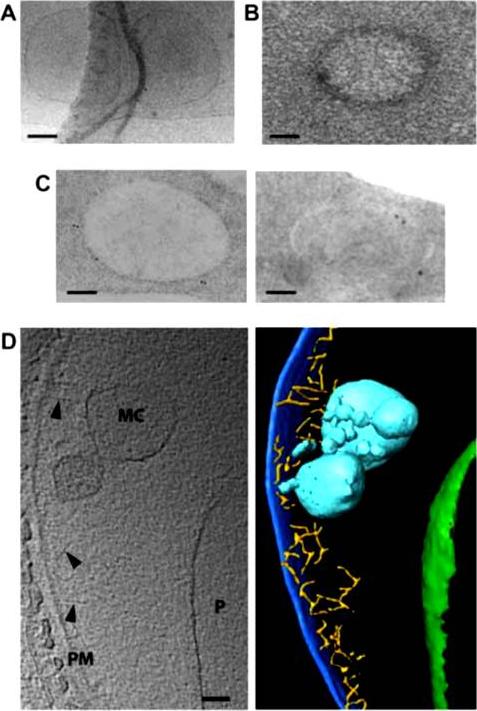
Morphology of young Maurer’s clefts in ring-infected HbAA erythrocytes. Representative electron-microscopic images of parasitized erythrocytes preserved by: A. cryo-preservation; B. high pressure freezing and freeze-substitution. Post staining with uranyl acetate revealed a densely packed protein coat surrounding the membrane of the Maurer’s cleft. C. Immuno-electron micrograph of cells preserved by high pressure freezing and freeze substitution, and immuno-labelled with an antiserum to PfSBP1 coupled to 10 nm protein A colloidal gold. D. Section through a cyro-electron tomogram (left) and corresponding surface rendered view (right) of a Maurer’s cleft precursor in a ring-infected erythrocyte. Labeling and color code as following: PM, erythrocyte plasma membrane (dark blue); MC, Maurer’s cleft precursor (cyan); P, parasite (boundary of parasite, green), actin filaments (yellow) indicated by arrowheads. Scale bars, 100 nm in all images.Kilian N, Dittmer M, Cyrklaff M, Ouermi D, Bisseye C, Simpore J, Frischknecht F, Sanchez CP, Lanzer M. Hemoglobin S and C affect the motion of Maurer's clefts in P. falciparum-infected erythrocytes. Cell Microbiol. 2013 15(7):1111-26
See original on MMP
Post-embedding immunoelectron microscopy using 6B4 monoclonal. Panoramic view of an infected erythrocyte (A) and higher magnification images (B–D) showing the preferential location of gold particles over Maurer’s clefts. Panels (E) and (F) arephotographs of ‘parasite ghosts’ probed with 6B4, showing the reaction of the monoclonal antibody over cytoplasmic vesicles. Bar indicates 0.2 mm. Immunoelectron microscopy demonstrated that the monoclonal antibody reacts with cytoplasmic vesicles of Plasmodium falciparum infected erythrocyte referred to as Maurer’s clefts. 6B4 recognized 50 and 41 kDa antigens. The 50 kDa antigen is most probably PfSBP1.Martinez SL, Clavijo CA, Winograd E. Identification of peripheral membrane proteins associated with the tubo-vesicular network of Plasmodium falciparum infected erythrocytes. Mol Biochem Parasitol. 1998 91:273-80. Copyright Elsevier 2009.
See original on MMP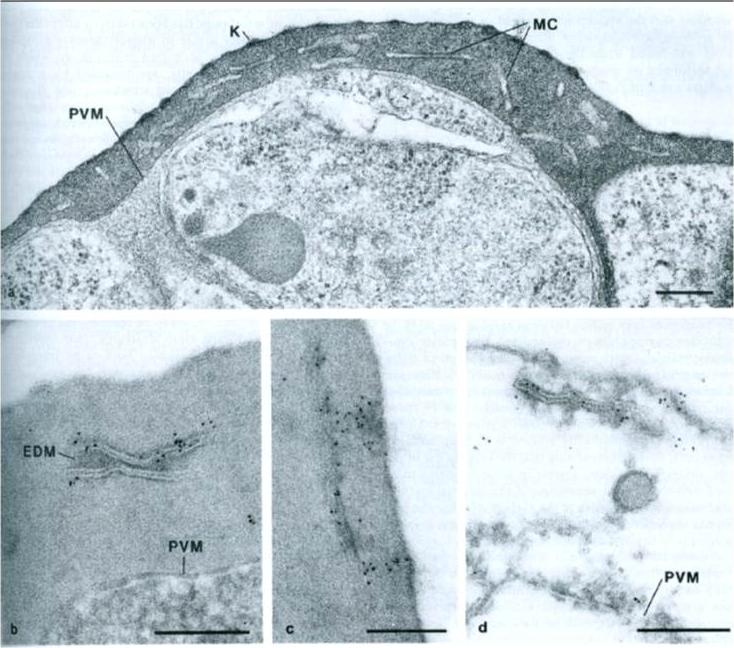
a) Morphology of and infected cell. PVM – parasitophorous vacuole membrane; MC – Maurer’s clefts; K – knob structures. b) immunolabeling showing gold particles over Maurer’s clafts. EDM – electron dense material. c) Localization of gold particles over Maurer’s clefts, adjacent to plasma membrane. d) Tris-lysed infected cell, gold particles distributed over membranes of Maurer’s clefts. Bar 200 nm.Etzion Z, Perkins ME. Localization of a parasite encoded protein to erythrocyte cytoplasmic vesicles of Plasmodium falciparum-infected cells. Eur J Cell Biol. 1989 48:174-9.
See original on MMP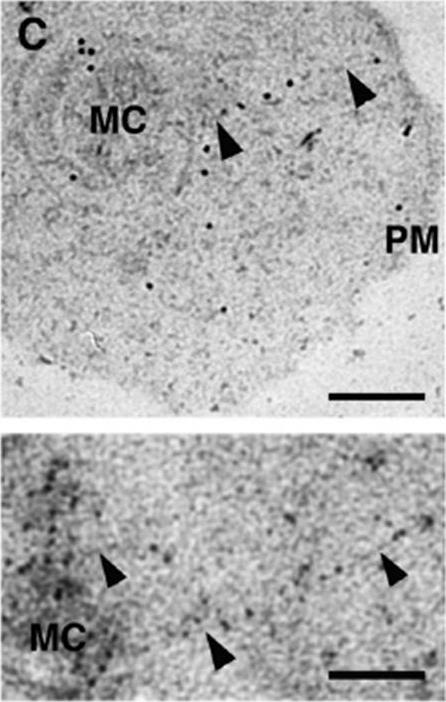
Immunolabeling of infected HbCC erythrocyte, using an antiserum against the Maurer’s clefts marker PfSBP1. PfSBP1 identified the amorphous membrane conglomerates as aberrant Maurer’s clefts seen in P. falciparum–infected erythrocytes (at the trophozoite stage) containing homozygous HbCC.Cyrklaff M, Sanchez CP, Kilian N, Bisseye C, Simpore J, Frischknecht F, Lanzer M. Hemoglobins S and C Interfere with Actin Remodeling in Plasmodium falciparum-Infected Erythrocytes. Science. 2011 334(6060):1283-6
See original on MMP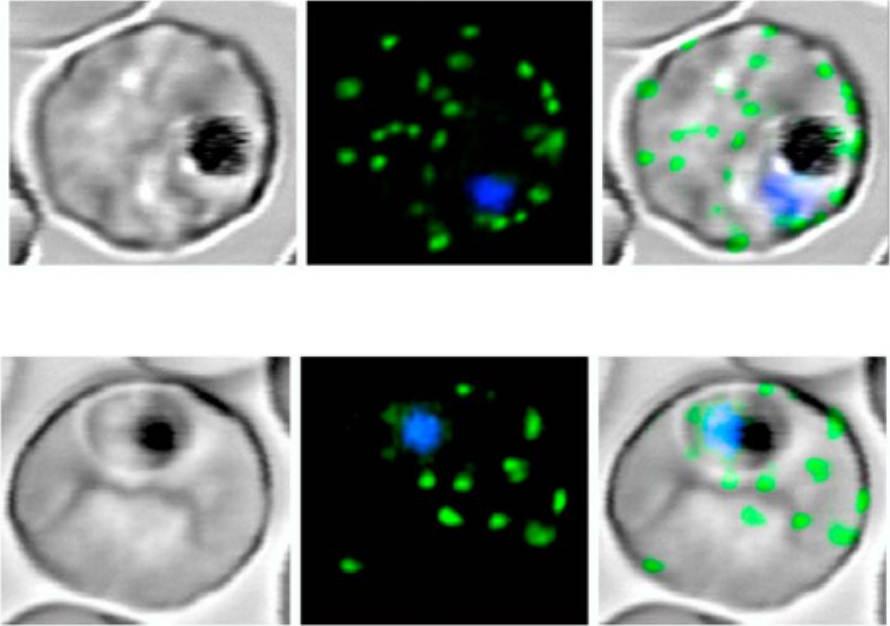
Live P. falciparum-infected erythrocytes were incubated in physiological Ringer’s solution and viewed, at 37 oC, using an LSM510 confocal laser scanning microscope. Confocal images of live P. falciparum-infected erythrocytes expressing SBP. Left images, differential interference contrast (DIC); middle images, GFP fluorescence and nuclear staining with Hoechst; right images, overlay. Scale bar, 4 μm.The right column shows confocal live cell images of P. falciparum-infected erythrocytes expressing MAHRP. Both proteins localize to the Maurer’s clefts.Saridaki T, Fröhlich KS, Breton CB, Lanzer M. Export of PfSBP1 to the Plasmodium falciparum Maurer's clefts. Traffic. 2008 10(2):137-52.
See original on MMP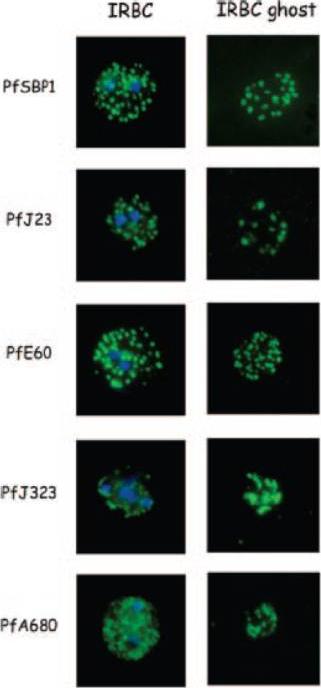
Indirect immunofluorescence of P. falciparum-infected erythrocytes and infected red blood cell (IRBC) ghosts. Air-dried infected red blood cells and infected red blood cell ghosts were incubated with mouse antibodies raised against GST fusion proteins as indicated. The nuclei were stained with DAPI (blue). Negative controls were performed using preimmune sera and anti-GST antibodies (not shown). All proteins are associated with Maurer;s clefts.Vincensini L, Richert S, Blisnick T, Van Dorsselaer A, Leize-Wagner E, Rabilloud T, Braun Breton C. Proteomic analysis identifies novel proteins of the Maurer's clefts, a secretory compartment delivering Plasmodium falciparum proteins to the surface of its host cell. Mol Cell Proteomics. 2005 4:582-93.
See original on MMP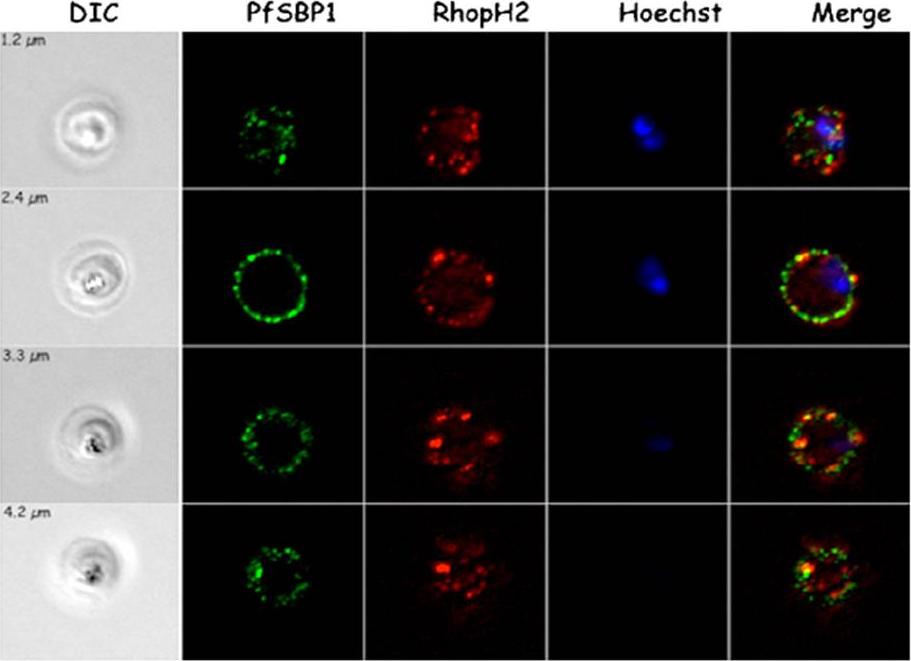
Localization of RhopH2 vs. PfSBP1 in trophozoites. Immunofluorescence studies on SLO-permeabilized trophozoites using RhopH2-specific sera and mouse PfSBP1-specific serum as indicated. The differential interference contrast (DIC) images, the Hoechst nuclei staining and the merge are presented. Four acquisitions at different z-value (as indicated) are presented in each panel. PfSBP1 localizes to the Maurer’s clefts.Vincensini L, Fall G, Berry L, Blisnick T, Braun Breton C. The RhopH complex is transferred to the host cell cytoplasm following red blood cell invasion by Plasmodium falciparum. Mol Biochem Parasitol. 2008 160:81-9. Copyright Elsevier 2009.
See original on MMP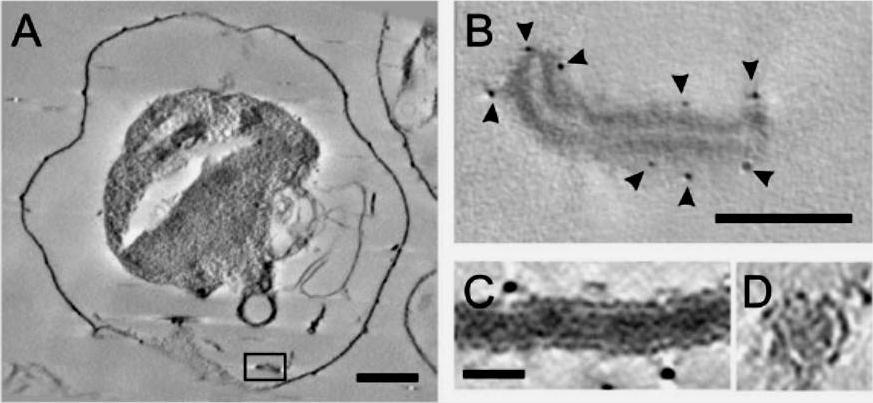
Electron tomography of individual Maurer’s cleft bodies, tubules and vesicles in a Plasmodium falciparum strain 3D7-infected red blood cell (RBC). (A) A virtual section with an individual Maurer’s cleft marked with a rectangle, which is depicted at higher resolution in (B). The presence of the gold particles (arrowheads) indicates labelling with antibodies recognising the Maurer’s cleft resident, skeleton-binding protein-1 (SBP1). (C and D) Virtual sections showing transverse (C) and cross-section (D) views through a tubular structure. Scale bars: (A) 1 mm; (B) 100 nm; (C and D) 25 nm..Hanssen E, Carlton P, Deed S, Klonis N, Sedat J, Derisi J, Tilley L. Whole cell imaging reveals novel modular features of the exomembrane system of the malaria parasite, Plasmodium falciparum. Int J Parasitol. 2010 40:123-134. Copyright Elsevier 2010.
See original on MMP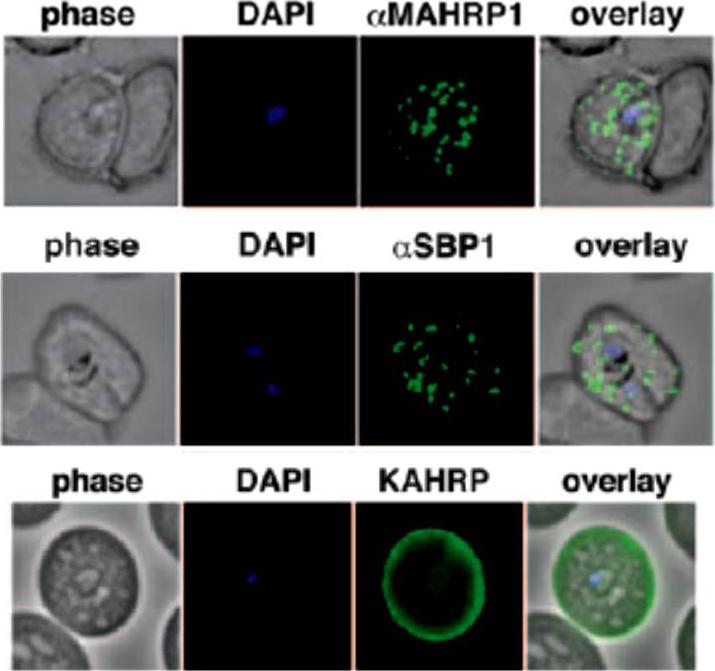
Localization of the resident Maurer clefts markers PfSBP1 and PfMAHRP1 in CS2. Both proteins trafficked to Maurer’s clefts. KAHRP is trafficked normally to the erythrocyte membrane and assembled into knob structures.Maier AG, Rug M, O'Neill MT, Beeson JG, Marti M, Reeder J, Cowman AF. Skeleton-binding protein 1 functions at the parasitophorous vacuole membrane to traffic PfEMP1 to the Plasmodium falciparum-infected erythrocyte surface. Blood. 2007 109:1289-97.
See original on MMP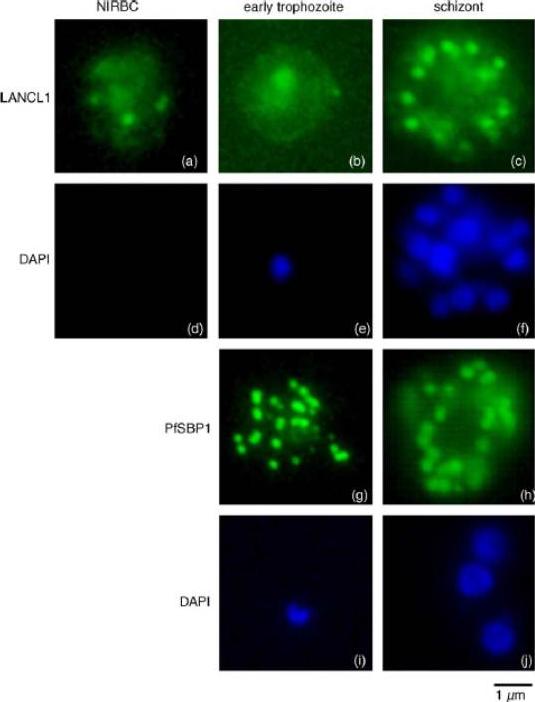
Immunofluorescence localisation of LANCL1 in uninfected and P. falciparum-infected erythrocytes. Un-infected erythrocytes and P. falciparum-infected erythrocytes at different stages of the parasite intra-erythrocytic cycle were fixed and permeabilized. Immunofluorescence analyses were performed using the rabbit H60 LANCL1-specific serum, and a mouse serum raised against the carboxy-terminal domain of PfSBP1. DAPI staining of the nuclei assessed the stage of the intra-erythrocytic parasite (in blue). In uninfected erythrocytes (a) and early trophozoites (b) a pattern consistent with a cytosolic localization and spots of aggregation of LANCL1 is observed. In P. falciparum schizonts (c), an additional labelling similar to that obtained with PfSBP1-specific antibodies (g and h) is observed, suggesting a Maurer’s cleft-like localization of LANCL1.Blisnick T, Vincensini L, Barale JC, Namane A, Braun Breton C. LANCL1, an erythrocyte protein recruited to the Maurer's clefts during Plasmodium falciparum development. Mol Biochem Parasitol. 2005 141:39-47. Copyright Elsevier 2010.
See original on MMP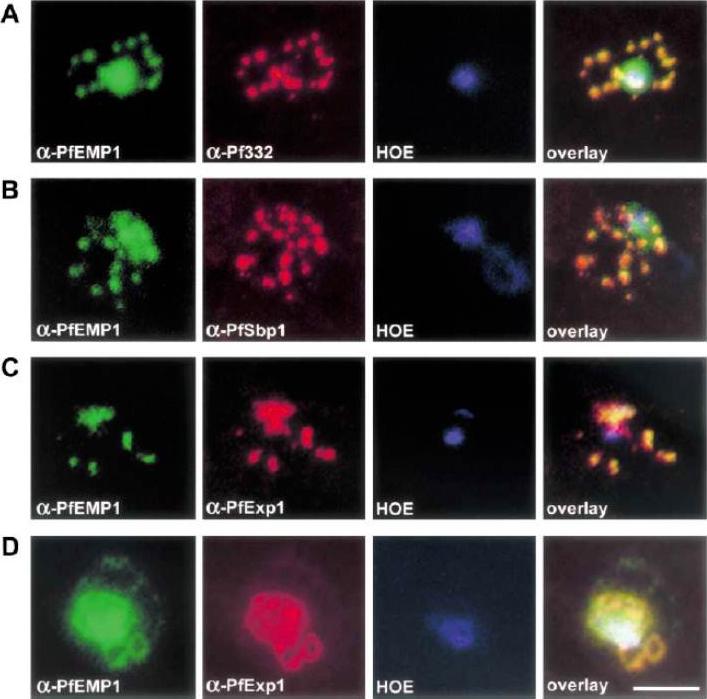
Co-localization of PfEMP1 with Pf332, PfSbp1 and PfExp-1 in P. falciparum-infected erythrocytes. Double immuno-fluorescence images of infected erythrocytes at the trophozoite stage with antibodies against: (A) PfEMP1 (a-PfEMP1) and Pf332 (a-Pf332); B) PfEMP1 (a-PfEMP1) and PfSbp1 (a-PfSbp1); (C, D) PfEMP1 (a-PfEMP1) and PfExp-1 (a-PfExp1). Different morphological appearances of the structures stained with antibodies against PfEMP1 and PfExp-1 are shown in (C and D). (HOE), DNA staining by Hoechst. (overlay), Overlay of the micrographs shown in a row. Representative examples of three independent experiments are shown. Bar, 5 mm.Wickert H, Wissing F, Andrews KT, Stich A, Krohne G, Lanzer M. Evidence for trafficking of PfEMP1 to the surface of P. falciparum-infected erythrocytes via a complex membrane network. Eur J Cell Biol. 2003 82:271-84.
See original on MMP
Co-localization of variant surface antigens (VSA) during the intraerythrocytic developmental cycle. A, B: Co-localization of PfEMP1, RIFIN, STEVOR and PfMC-2TM (green) with marker proteins for the erythrocyte membrane (spectrin), the Maurer’s clefts (SBP1) and the merozoite surrounding membrane (MSP1) (red). Subcellular VSA localization was determined in trophozoites (A) as well as in schizonts and free merozites (B) from clinical isolates as well as strain 3D7. Nuclei were stained with DAPI (blue). In the trophozoite stage, RIFIN proteins were exported predominantly to the Maurer’s clefts and the erythrocyte membrane, whereas STEVOR and PfMC-2TM proteins seemed to localize predominantly to the IE membrane. A-type RIFINs, STEVORs and PfMC-2TMs were located in close proximity to the nuclei in dividing parasites in the clinical isolates. In strain 3D7 schizonts, PfMC-2TM family proteins exhibited two distinct localization patterns depending on the antisera used for detection. PfMC-2TM proteins appeared to associate with the parasitophorous vacuole membrane or parasite membrane when using a-PfMC-2TM-SC, while staining with a-PfMC-2TM-CT resulted in a fluorescence pattern similar to RIFIN and STEVOR.Bachmann A, Petter M, Tilly AK, Biller L, Uliczka KA, Duffy MF, Tannich E, Bruchhaus I. Temporal Expression and Localization Patterns of Variant surface Antigens in Clinical Plasmodium falciparum Isolates during Erythrocyte Schizogony. PLoS One. 2012;7(11): e49540.
See original on MMP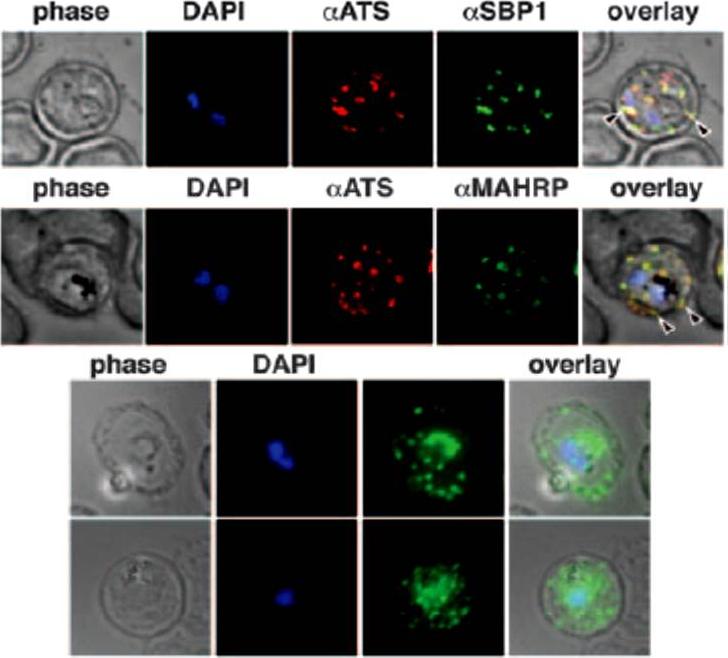
Immunofluorescence study to assess trafficking of PfEMP1 in erythrocytes infected with CS2 parasites. A. Cells were probed with anti-ATS (anti-PfEMP1) and anti-SBP1 antibodies showed typical PfEMP1 localization in Maurer clefts in the erythrocyte, B. Colocalization with anti-ATS and anti-MAHRP antibodies to Maurer clefts. C. Expression of PfEMP1-YFP in CS2-infected erythrocytes are shown by anti-GFP labeling. The fluorescence is localized in the parasite, but the majority of the protein is transported into the erythrocyte cytosol and shows a characteristic Maurer clefts staining.Maier AG, Rug M, O'Neill MT, Beeson JG, Marti M, Reeder J, Cowman AF. Skeleton-binding protein 1 functions at the parasitophorous vacuole membrane to traffic PfEMP1 to the Plasmodium falciparum-infected erythrocyte surface. Blood. 2007 109:1289-97. >
See original on MMP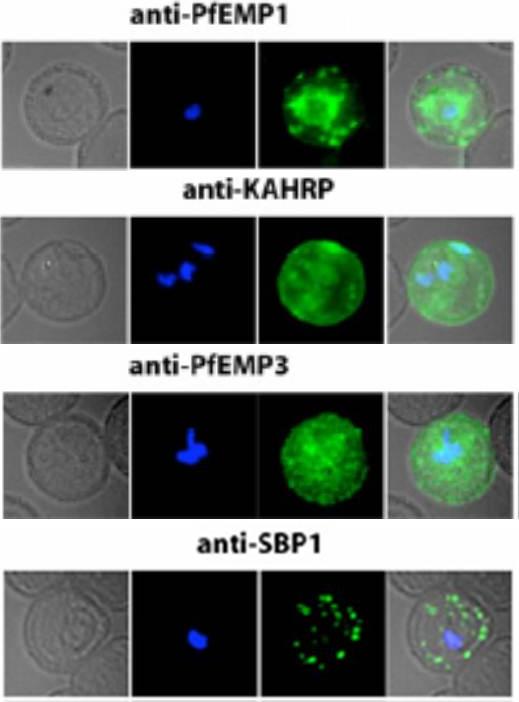
Localization of PfEMP1, KAHRP, PfEMP3 and SBP1 in P. falciparum-infected erythrocytes. The first column of each panel shows a bright-field image, the second panel specific antibody and the third panel overlay of the previous two and a nuclear stain (DAPI). PfEMP1 displays and functions on the surface of infected erythrocytes. KAHRP and PfEMP3 are exported to the erythrocyte cytoplasm and interacts with Maurer’s clefts and eventually reach the inner side of the host cell membrane. SBP1 is a Maurer’s clefts resident protein.Maier AG, Rug M, O'Neill MT, Brown M, Chakravorty S, Szestak T, Chesson J, Wu Y, Hughes K, Coppel RL, Newbold C, Beeson JG, Craig A, Crabb BS, Cowman AF. Exported proteins required for virulence and rigidity of Plasmodium falciparum-infected human erythrocytes. Cell. 2008 134:48-61.
See original on MMP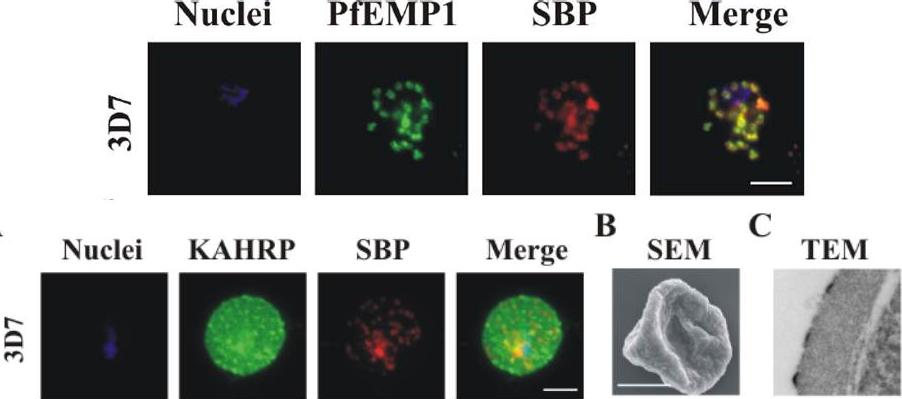
Upper row: PfEMP1 traffics to the Maurer’s clefts. Immunofluorescence microscopy of acetone-fixed RBCs infected with 3D7 parasites probed with anti-PfEMP1 ATS antiserum (green) and anti-SBP1 (red) – top pictures, or with anti-KAHRP antiserum (green) and anti-SBP1 (red) – bottom picture. The nuclei were visualized with DAPI and parasites with a single nucleus were chosen for analysis. The data are typical of at least 30 cells examined for each parasite line. Scale bar: 5 μm. PfEMP1 accumulates at the Maurer’s clefts as confirmed by co-labelling with the resident Maurer’s cleft marker, SBP1.Lower row: Immunofluorescence microscopy of acetone-fixed RBCs infected with 3D7 parasites probed with anti-KAHRP antiserum (green) and anti-SBP1 (red) and co-stained with DAPI (blue). Scale bar: 5 μm. KAHRP forms the main structural component of the knobs at the infected RBC surface in which PfEMP1 is concentrated. An anti-KAHRP antiserum gave a roughly homogenous staining pattern at the surface of RBCs infected with wild type 3D7.Dixon MW, Kenny S, McMillan PJ, Hanssen E, Trenholme KR, Gardiner DL, Tilley L. Genetic ablation of a Maurer's cleft protein prevents assembly of the Plasmodium falciparum virulence complex. Mol Microbiol. 2011 81(4):982-93
See original on MMP
Transgenic parasites were fixed using acetone and detected with anti-GFP and anti-SBP1 antibodies. The panel shows REX-C6 colocalizations, GFP fluorescence of the chimeric protein in green and SBP1 fluorescence in red. The merge panel on the right shows complete colocalization of the two cleft proteins, confirming the presence of REX–GFP at the Maurer’s clefts.Dixon MW, Hawthorne PL, Spielmann T, Anderson KL, Trenholme KR, Gardiner DL. Targeting of the ring exported protein 1 to the Maurer's clefts is mediated by a two-phase process. Traffic. 2008 9:1316-26.
See original on MMP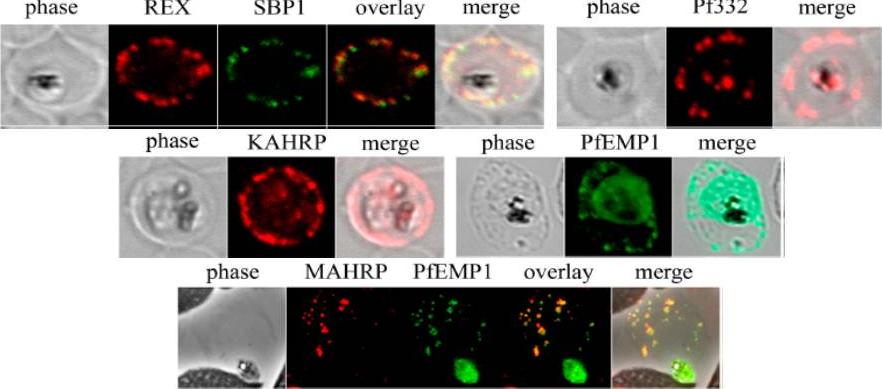
Analysis of expression and localization of Maurer’s cleft–associated proteins in IRBCs. Confocal immunofluorescence analysis of resident (REX-PFI1735c, SBP1-PFE0065w, and Pf332-PF11_0507) or transiently associated (KAHRP-PFB0100c and PfEMP1) Maurer’s cleft proteins. Colocalization of the resident Maurer’s cleft marker MAHRP-MAL13P1.413 with PfEMP1 in streptolysin O–pretreated IRBCs demonstrates that PfEMP1 is associated with Maurer’s clefts.Cooke BM, Buckingham DW, Glenister FK, Fernandez KM, Bannister LH, Marti M, Mohandas N, Coppel RL. A Maurer's cleft-associated protein is essential for expression of the major malaria virulence antigen on the surface of infected red blood cells. J Cell Biol. 2006 172:899-908.
See original on MMP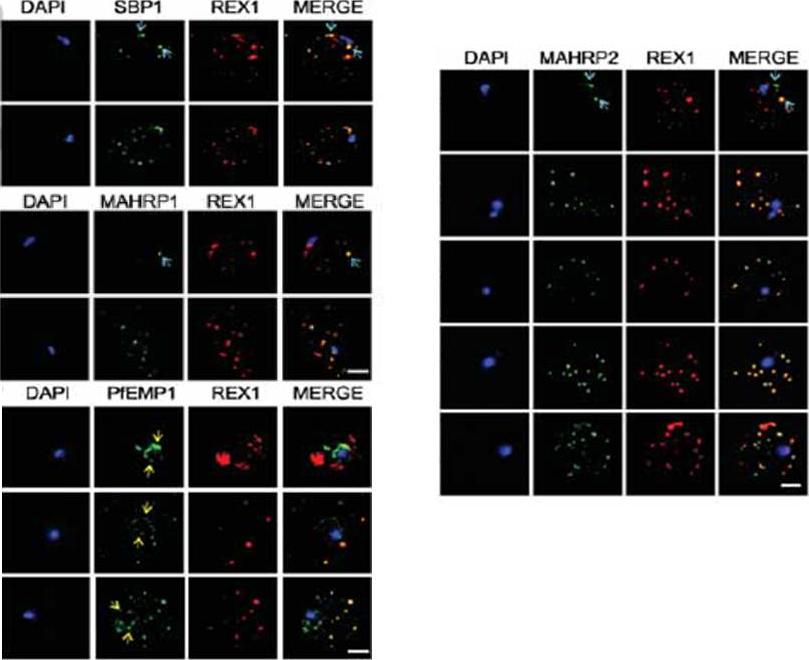
Immunofluorescence microscopy showing staggered delivery of different exomembrane components. (A-D) Infected RBCs were synchronized to a 1 h window, samples were collected at 2 h intervals and smears were fixed with acetone and stained with antibodies recognizing REX1, MAHRP1, SBP1, MAHRP2 and PfEMP1 (ATS) and co-stained with DAPI. Images are presented at time points before and after delivery to the RBC cytoplasm. Scale bars = 3 μm. McMillan PJ, Millet C, Batinovic S, Maiorca M, Hanssen E, Kenny S, Muhle RA, Melcher M, Fidock DA, Smith JD, Dixon MW, Tilley L. Spatial and temporal mapping of the PfEMP1 export pathway in Plasmodium falciparum. Cell Microbiol. 2013 15(8):1401-18
See original on MMP
Export of membrane proteins is independent of cleft formation. IFA showing that in REX2-GFPcrt-expressing parasites (e) and 3D7 (f), early clefts contain REX1 and but not REX2-GFPcrt (e, upper panels) or RIFIN A29 (RIF-A29, f, upper panels). On progress of the culture to the trophozoite stage, the clefts containing REX1 and now also contain REX2-GFPcrt (e, lower panel) and RIFIN A29 (f, lower panel). Dots positive for RIFIN A29 only, found in ~50% of cells, are indicated by white arrows. Scale bars, 5 μm; h.p.i., hours post invasion. the clefts in early stages contained only the early markers (e,f). In contrast, later stages were positive for all four antigens (e,f). Crucially, in later stages all antigens occupied the same clefts (e,f), demonstrating that the clefts initially containing REX1 and SBP1 accumulated the other proteins later on. This establishes that export occurred without cleft formation.Grüring C, Heiber A, Kruse F, Ungefehr J, Gilberger TW, Spielmann T. Development and host cell modifications of Plasmodium falciparum blood stages in four dimensions. Nat Commun. 2011 2:165.
See original on MMP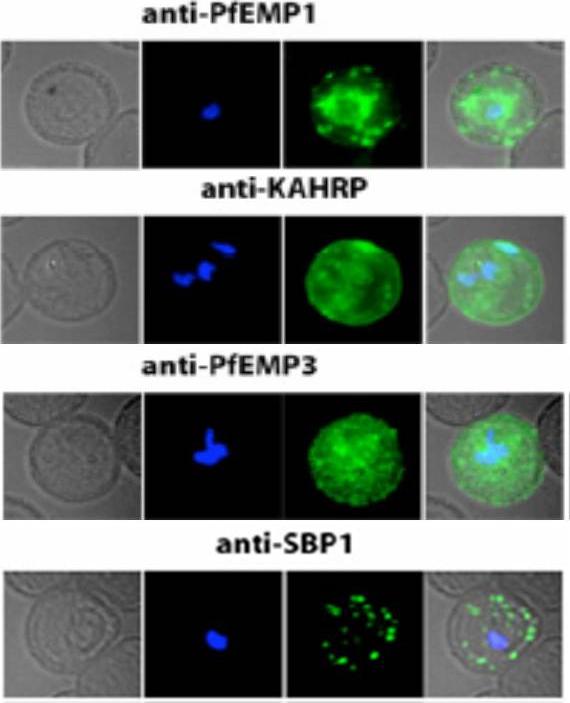
Localization of PfEMP1, KAHRP, PfEMP3 and SBP1 in P. falciparum-infected erythrocytes. The first column of each panel shows a bright-field image, the second panel specific antibody and the third panel overlay of the previous two and a nuclear stain (DAPI). PfEMP1 displays and functions on the surface of infected erythrocytes. KAHRP and PfEMP3 are exported to the erythrocyte cytoplasm and interacts with Maurer’s clefts and eventually reach the inner side of the host cell membrane. SBP1 is a Maurer’s clefts resident protein.Maier AG, Rug M, O'Neill MT, Brown M, Chakravorty S, Szestak T, Chesson J, Wu Y, Hughes K, Coppel RL, Newbold C, Beeson JG, Craig A, Crabb BS, Cowman AF. Exported proteins required for virulence and rigidity of Plasmodium falciparum-infected human erythrocytes. Cell. 2008 134:48-61.
See original on MMP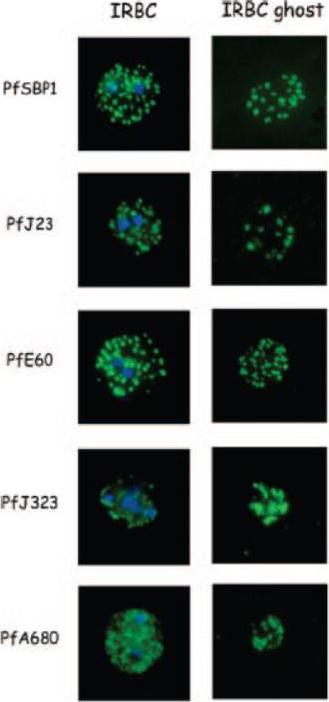
Indirect immunofluorescence of P. falciparum-infected erythrocytes and infected red blood cell (IRBC) ghosts. Air-dried infected red blood cells and infected red blood cell ghosts were incubated with mouse antibodies raised against GST fusion proteins as indicated. The nuclei were stained with DAPI (blue). Negative controls were performed using preimmune sera and anti-GST antibodies (not shown). All proteins are associated with Maurer;s clefts.Vincensini L, Richert S, Blisnick T, Van Dorsselaer A, Leize-Wagner E, Rabilloud T, Braun Breton C. Proteomic analysis identifies novel proteins of the Maurer's clefts, a secretory compartment delivering Plasmodium falciparum proteins to the surface of its host cell. Mol Cell Proteomics. 2005 4:582-93.
See original on MMP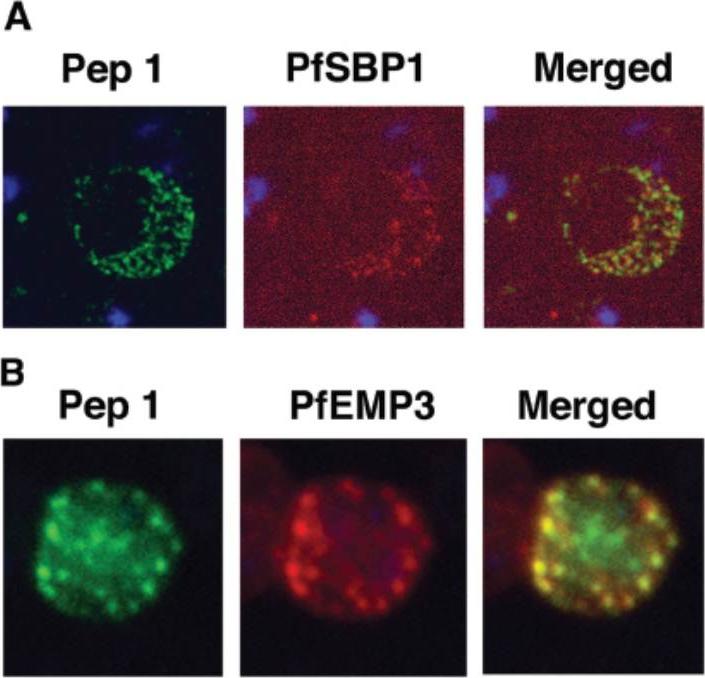
Colocalization studies with anti-peptide 1 antibodies and fixed 3D7 pRBC from schizont ghosts and PfEMP3 truncated 3D7 cell lines. (A) Schizont ghosts. (B) PfEMP3 truncated 3D7 cell lines. Parasite nuclei were stained with DAPI (blue). Double staining was carried out with anti-peptide 1 rabbit serum (green) and mouse serum (B28) against the 77-kDa carboxy terminus of MC protein PfSBP1 (red) or mouse serum (DG662) against the carboxy terminus of PfEMP3 (red). Anti-peptide 1 serum was detected by using FITC-conjugated goat anti-rabbit IgG. Colocalizing antibody was detected by using Texas red-conjugated goat anti-mouse IgG. Magnification, x1,000. A truncated form of PfEMP-3 obtained by transfection was no longer distributed around the cytoplasmic surface of the erythrocyte membrane but was instead associated with structures believed to be MC. IFA experiments with antibodies against PfEMP-3 and STEVOR showed the colocalization of truncated PfEMP-3 and STEVOR (B), further supporting the observation that STEVOR is located in MC.Kaviratne M, Khan SM, Jarra W, Preiser PR. Small variant STEVOR antigen is uniquely located within Maurer's clefts in Plasmodium falciparum-infected red blood cells. Eukaryot Cell. 2002 1:926-35.
See original on MMP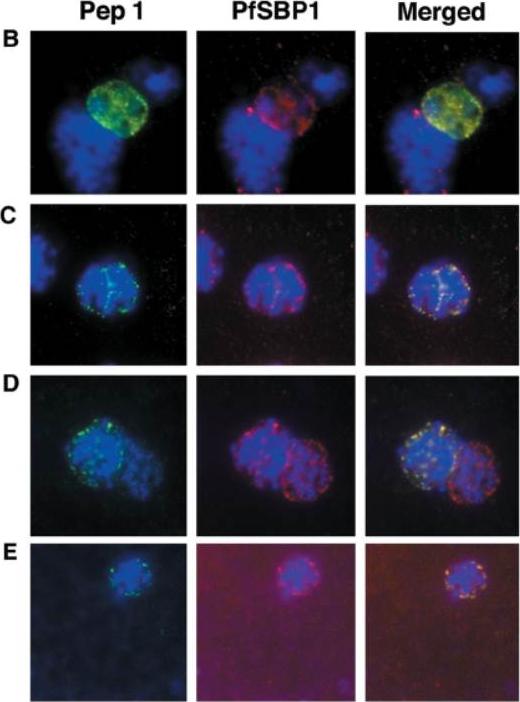
Colocalization studies with anti-peptide 1 antibodies and fixed 3D7 pRBCs (late trophozoites and schizonts). Parasite nuclei were stained with DAPI (blue). Double staining was carried out with MAb 61.2 against a 52-kDa rhoptry protein (red) and anti-peptide 1 serum (green) (A) and with mouse serum (B28) against the 77-kDa carboxy terminus of MC protein PfSBP1 (red) and anti-peptide 1 serum (STEVOR; green) (B to E). Schizonts progressd from binucleate to multinucleate in panels B to E, as determined by staining of nuclear material. Anti-peptide 1 serum was detected by using FITC conjugated goat anti-rabbit IgG. Colocalizing antibody was detected by using Texas red-conjugated goat anti-mouse IgG. Magnification, x1,000. Antibodies against STEVOR recognize proteins of the expected size (37 kDa) and localize STEVOR in Maurer’s clefts, unique membranous structures located in the cytoplasm of infected erythrocytes. Kaviratne M, Khan SM, Jarra W, Preiser PR. Small variant STEVOR antigen is uniquely located within Maurer's clefts in Plasmodium falciparum-infected red blood cells. Eukaryot Cell. 2002 1:926-35.
See original on MMP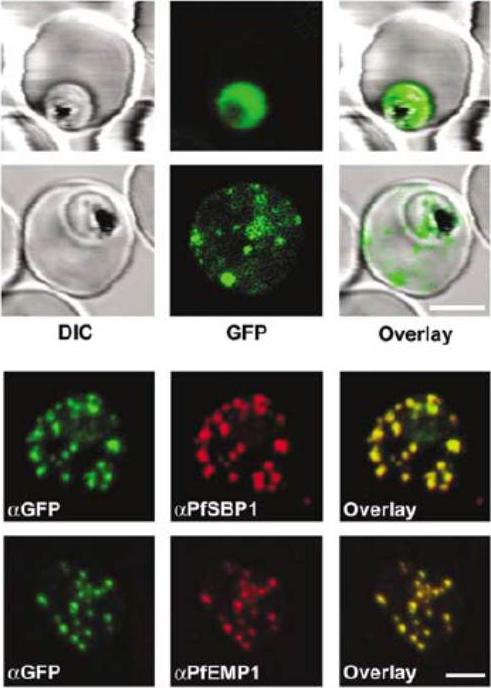
Subcellular localization of STEVORfull and GFP in P. falciparum-infected erythrocytes. (A) The upper row represents control (GFP alone) and the lower row STEVORfull. The left image shows differential interference contrast (DIC), middle image GFP fluorescence and the right image overlay. The GFP-only control reveals fluorescence only within the boundaries of the parasite plasma membrane. STEVORfull exhibits a dotted fluorescence pattern within the host erythrocyte cytoplasm, characteristic of the Maurer’s clefts. Bar, 4 mm. (B) Colocalization of STEVORfull (aGFP) with PfSBP1 (aPfSBP1) and/or PfEMP1 (aPfEMP1) by immunofluorescence microscopy. Overlay of signals is shown in the right panel. Bar, 3 mm.Przyborski JM, Miller SK, Pfahler JM, Henrich PP, Rohrbach P, Crabb BS, Lanzer M. Trafficking of STEVOR to the Maurer's clefts in Plasmodium falciparum-infected erythrocytes. EMBO J. 2005 24:2306-17. Copyright Nature Publishing Group 2011.
See original on MMP
Immunofluorescence analysis of red blood cells infected with parental (3D7) or FIKK4.2 knockout (1E2) parasites. Experiments were performed on infected red blood cells that had been synchronised at either the young ring stage or the more mature trophozoite/schizont stage. Air-dried and fixed blood smears on glass slides were dual-labelled with both a-FIKK4.2 and a-SBP1 (Maurer’s cleft) antibodies. Merge and phase overlay images demonstrate clearly that FIKK4.2 and SBP1 do not co-localise in infected red blood cells. Specific punctate fluorescence was observed in the cytoplasm of RBCs infected with ring-stage 3D7 parasites. A similar pattern was observed in RBCs infected with trophozoite/schizont-stage parasites, and although the pattern of fluorescence was more diffuse, individual puncta were still clearly discernible. The puncta appeared too small and too numerous to be Maurer’s clefts, and did not co-localise with either the classical Maurer’s cleft marker SBP1.Kats LM, Fernandez KM, Glenister FK, Herrmann S, Buckingham DW, Siddiqui G, Sharma L, Bamert R, Lucet I, Guillotte M, Mercereau-Puijalon O, Cooke BM. An exported kinase (FIKK4.2) that mediates virulence-associated changes in Plasmodium falciparum-infected red blood cells. Int J Parasitol. 2014 Feb 14. [Epub ahead of print]
See original on MMP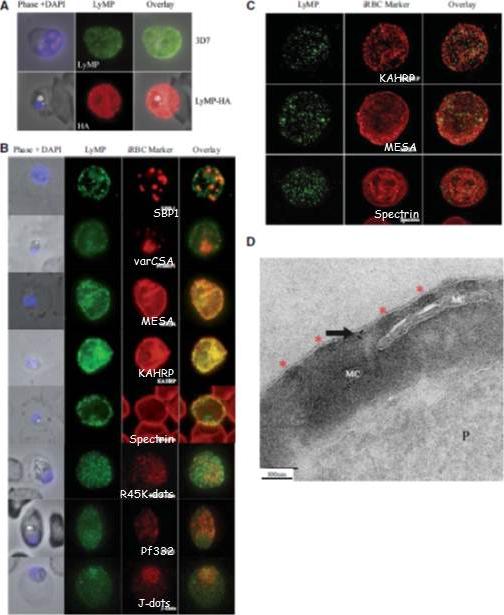
Localization of LyMP in iRBCs. A) Localization of LyMP in trophozoites, with either anti-LyMP (green) or anti-HA (red) showing that LyMP is exported into the RBC cytosol. 4’,6’-Diamidino-2-phenylidole (DAPI) is a nuclear stain (blue). B) Colocalization of LyMP (green) with a variety of iRBC markers (red) that are indicated in the panel. DAPI is a nuclear stain (blue). C) High-resolution structured illumination fluorescence microscopy (OMX) with anti-LyMP (green) and either anti-KAHRP, anti-MESA, or anti-spectrin (red) colocalizations. Corresponding overlay images are shown for all. D) Immunoelectron micrograph of gold-labeled Ha-tagged parasites. Red asterisks indicate knobs and the arrow indicates gold labeling of LyMP at the RBC membrane skeleton. P, parasite; MC, Maurer’s clefts. LyMP showed a pattern of distribution similar to that for MESA, KAHRP. LyMP did not consistently colocalize with MCs or to other punctate structures previously identified in the iRBC cytosol such as those seen for FIKK4.2 (R45) or for J-dots (KAHsp40).Proellocks NI, Herrmann S, Buckingham DW, Hanssen E, Hodges EK, Elsworth B, Morahan BJ, Coppel RL, Cooke BM. A lysine-rich membrane-associated PHISTb protein involved in alteration of the cytoadhesive properties of Plasmodium falciparum-infected red blood cells. FASEB J. 2014 Apr 4. [Epub ahead of print]
See original on MMP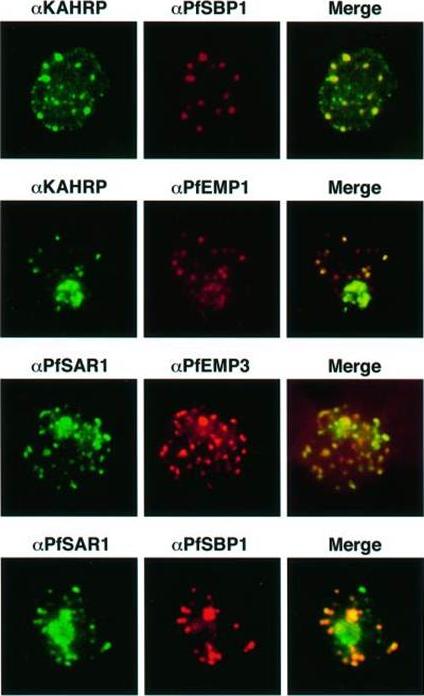
Transient co-localization of KAHRP, PfSBP1, PfEMP1, PfEMP3 and PfSAR1p to Maurer’s clefts in ring stage P.falciparum-infected erythrocytes. First row: co-localization of KAHRP (green) with PfSBP1 (red). Second row: co-localization of KAHRP (green) with PfEMP1 (red). Third row: co-localization of PfSAR1p (green) with PfEMP3 (red). Fourth row: co-localization of PfSAR1p (green) with PfSBP1 (red). The third window in each row represents the merging of the red and green channels. Yellow areas represent regions of co-localization.Wickham ME, Rug M, Ralph SA, Klonis N, McFadden GI, Tilley L, Cowman AF. Trafficking and assembly of the cytoadherence complex in Plasmodium falciparum-infected human erythrocytes. EMBO J. 2001 20(20):5636-49.
See original on MMP
Immunofluorescence microscopy of paraformaldehyde-fixed RBCs infected with 3D7, SKO, TK1 or TK2 parasites probed with anti-REX166-169 antiserum and anti-SBP1. The nuclei were visualized with Hoechst staining. Bar is 5 mm. Maurer’s clefts are observed as punctate structures in the RBC cytoplasm in 3D7 parasites labelled with the known Maurer’s cleft protein, SBP1 or with the antiserum against REX166-169 (top row). By contrast, RBCs infected with D10 parasites are not labelled with either REX1 antiserum (data not shown). In the SKO (second row), the antiserum against REX166-169 also recognized the truncated REX1 protein at the Maurer’s clefts. However, an antiserum against the C-terminal region does not recognize the truncated REX1 protein. The Maurer’s clefts location was confirmed by dual labelling with anti-SBP1 antiserum (second row).Hanssen E, Hawthorne P, Dixon MW, Trenholme KR, McMillan PJ, Spielmann T, Gardiner DL, Tilley L. Targeted mutagenesis of the ring-exported protein-1 of Plasmodium falciparum disrupts the architecture of Maurer's cleft organelles. Mol Microbiol. 2008 69(4):938-53
See original on MMP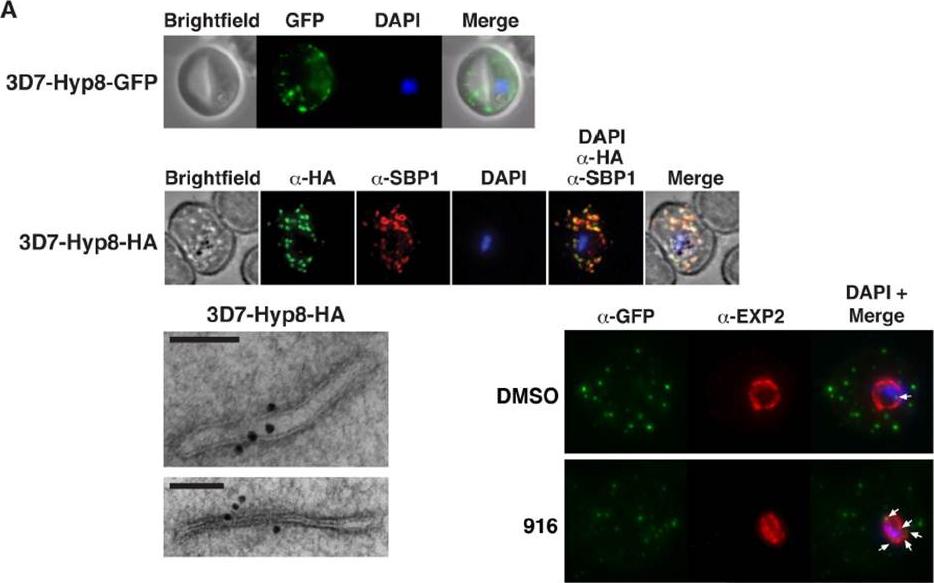
PMV inhibition impairs protein export, PfEMP1 display, and cytoadherence. (A) (Top) Immunofluorescent micrographs show Hyp8-GFP (exported protein hyp8),is exported and localizes within puncta in the infected erythrocyte. (Middle) Hyp8-HA localizes within SBP1-containing MCs. (Right) Immunoelectron microscopy shows Hyp8-HA localization at MCs. Scale bar is 100 nm. Immunofluorescence microscopy showed Hyp8-GFP is exported and colocalizes with SBP1 in MCs(B) Maximum intensity projection micrographs showing export of Hyp8-GFP to MCs and secretion of EXP2 to the parasitophorous vacuole membrane following treatment with DMSO or 916 (50 mM). Puncta of nonexported GFP within the parasite and vacuole is shown (arrows)Sleebs BE, Lopaticki S, Marapana DS, O'Neill MT, Rajasekaran P, Gazdik M, Günther S, Whitehead LW, Lowes KN, Barfod L, Hviid L, Shaw PJ, Hodder AN, Smith BJ, Cowman AF, Boddey JA. Inhibition of Plasmepsin V Activity Demonstrates Its Essential Role in Protein Export, PfEMP1 Display, and Survival of Malaria Parasites. PLoS Biol. 2014 12(7):e1001897.
See original on MMP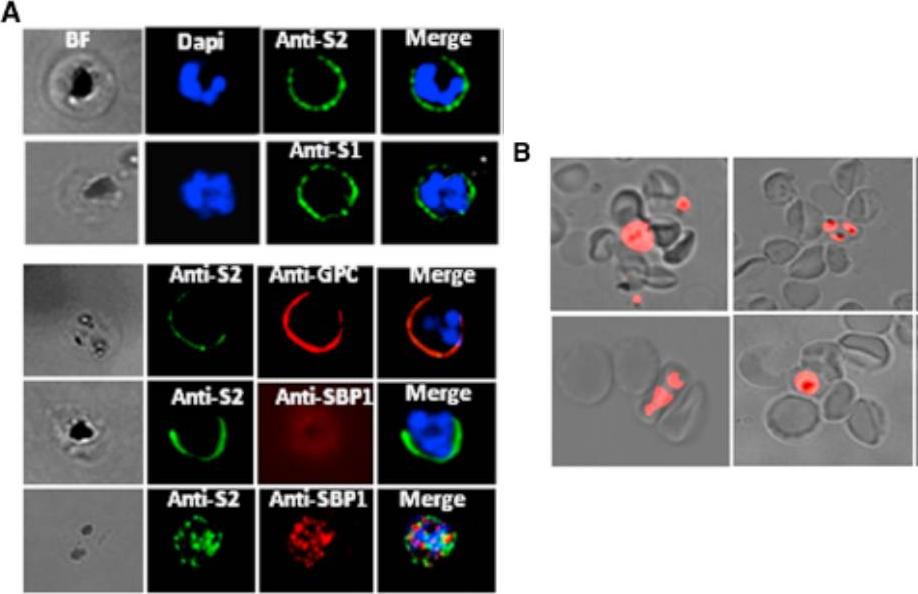
Analysis of STEVOR Expression in the 3D7DMAHRP1 Parasites -parasite line in which the disruption of the mahrp1 gene has been shown to compromise trafficking of PfEMP1 to the iRBC surface allowing any adhesion or binding phenotype to be attributed to parasite ligands other than PfEMP1. Live IFA with anti-S1 and anti-S2 (anti-STEVOR Abs) showed that trafficking of STEVOR to the 3D7DMAHRP1-iRBC surface was not impaired. (A) Detection of STEVOR on live 3D7DMAHRP1-R+ -iRBCs surface by anti-S1 and anti-S2 (top panel). Dual staining of live 3D7DMAHRP1-R+ -iRBCs with anti-S2 and anti-GPC antibodies (bottom panel, top row) or anti-SBP1 (bottom panel, middle row) illustrating the external location of both STEVOR and GPC (Glycophorin C). SBP1 internal location revealed by IFA on fixed parasites (bottom panel, bottom row). Only surface-expressed STEVOR was detected by live IFA of iRBCs, as staining with antibody against the internally located Maurer’s clefts protein SBP1 was negative while dual IFA on fixed parasites showed double labeling of STEVOR and SBP1(B) Live wet-preparation images showing successful rosetting enrichment of 3D7DMAHRP1 line.Niang M, Bei AK, Madnani KG, Pelly S, Dankwa S, Kanjee U, Gunalan K, Amaladoss A, Yeo KP, Bob NS, Malleret B, Duraisingh MT, Preiser PR. STEVOR Is a Plasmodium falciparum Erythrocyte Binding Protein that Mediates Merozoite Invasion and Rosetting. Cell Host Microbe. 2014 16(1):81-93. PMID: 25011110
See original on MMP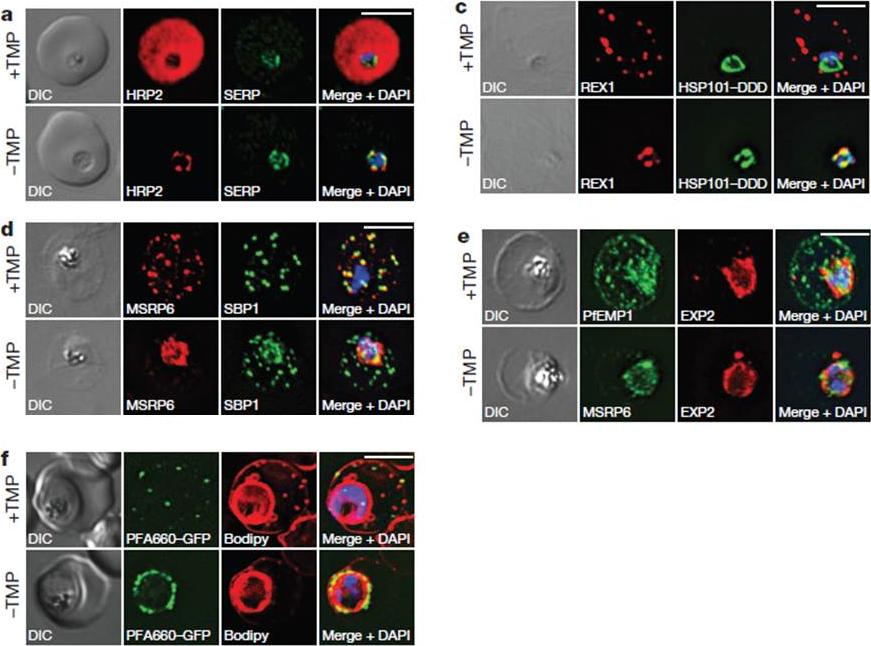
HSP101 is required for export of PEXEL and PNEP proteins. a, c, Immunofluorescence assay (IFA) of ring-stage 13F10 parasites with orwithout TMP (DDD-stabilizing small molecule trimethoprim). TMP was removed in late schizont stage and parasites were allowed to reinvade and grow 18–24 h before fixation with paraformaldehyde (a) or acetone (c). a, IFA of the exported PEXEL-containing protein HRP2. c, IFAs of the PNEP REX1, which colocalizes with HSP101DDD at the PVM in the absence of TMP. d, e, IFA of trophozoite-stage 13F10 parasites with or withoutTMP. TMP was removed in late ring stage and parasites were allowed to develop 12–24 h before fixation with acetone. f, Live fluorescence imaging of 13F10 parasites expressing a PFA660–GFP fusion and labelled with Bodipy TR Ceramide to demarcate the PVM (other membranes are also labelled). TMP treatment as in d, e. All scale bars, 5 mm. Images in c–f are representative of two independent experiments.Beck JR, Muralidharan V, Oksman A, Goldberg DE. PTEX component HSP101 mediates export of diverse malaria effectors into host erythrocytes. Nature. 2014 Jul 16. [Epub ahead of print]
See original on MMP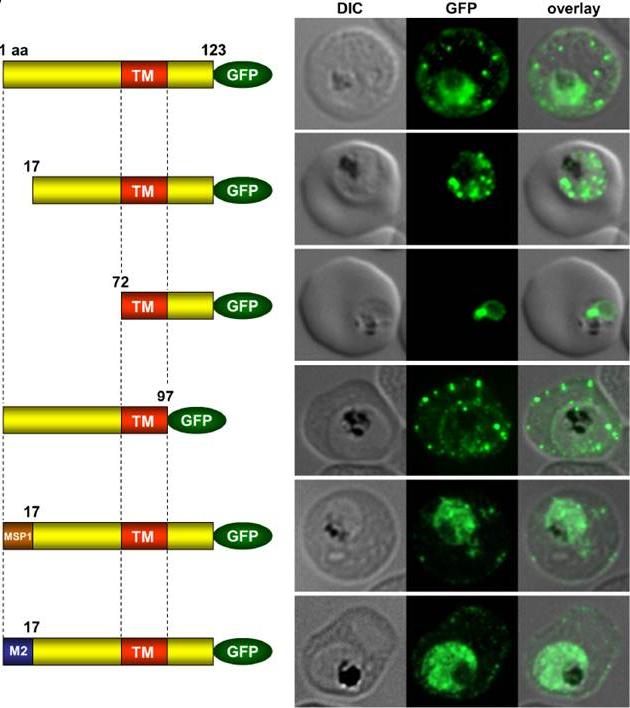
Immunofluorescence assays of MeOH-fixed RBCs infected with SEMP1-KO parasites expressing full-length and truncated or mutated forms of SEMP1 C-terminally fused to GFP. Expressed SEMP1 was labelled with rabbit a-GFP antibodies. The transmembrane domain is depicted in red (TM), a MSP1 signal peptide in brown (MSP1) and the MAHRP2 N-terminus in blue (M2). deletion of the complete C-terminus of SEMP1 (SEMP11–97-GFP) did not impair export and resulted in correct localisation whilst deletion of the first 16 amino acids (SEMP117–123-GFP) of the N-terminus resulted in an impaired export from the parasite. The deletion of 71 amino acids (SEMP172–123-GFP) of the N-terminus prevented export and seemed to have resulted in concentration of SEMP1 in or at the endoplasmic reticulum. If the first 16 amino acids of SEMP1 were replaced by the corresponding amino acids of MAHRP2 (M21–16SEMP117–123-GFP) or the classical signal peptide of MSP1 (MSP11–16SEMP117–123-GFP) the fusion protein was exported in both cases albeit with lower efficiency.Dietz O, Rusch S, Brand F, Mundwiler-Pachlatko E, Gaida A, Voss T, Beck HP. Characterization of the Small Exported Plasmodium falciparum Membrane Protein SEMP1. PLoS One. 2014 9(7):e103272.
See original on MMP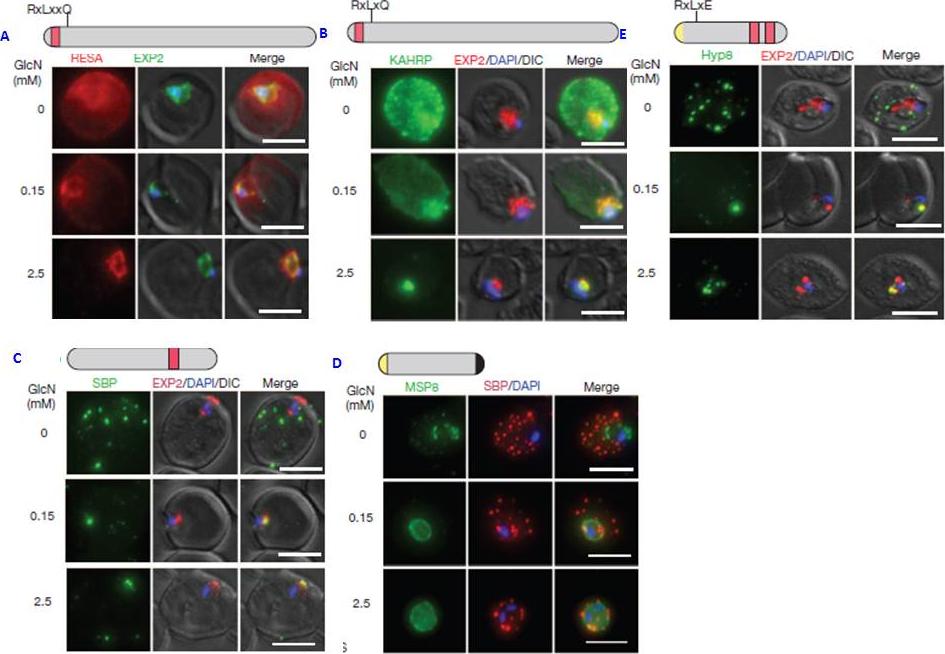
PTEX150 knockdown blocks protein export in P. falciparum. a–e, IFAs (right) and graphs (left) showing a decrease in the export of RESA (a) and KAHRP (b) and of SBP1 (c) and Hyp8 (d) (Maurer’s clefts, MCs) cells for each antibody or GlcN concentration) but similar levels of MSP8 (e) after treatment with GlcN. Scale bars, 5 mm. A strong blockage in export was seen at both 2.5mM and 0.15mM glucosamine in PTEX150-HAglmS parasites (a, b, c and d). No effect on either MSP8 expression or localization to the parasite membrane was observed (e).Elsworth B, Matthews K, Nie CQ, Kalanon M, Charnaud SC, Sanders PR, Chisholm SA, Counihan NA, Shaw PJ, Pino P, Chan JA, Azevedo MF, Rogerson SJ, Beeson JG, Crabb BS, Gilson PR, de Koning-Ward TF. PTEX is an essential nexus for protein export in malaria parasites. Nature. 2014 Jul 16. [Epub ahead of print] PMID: 25043043
See original on MMP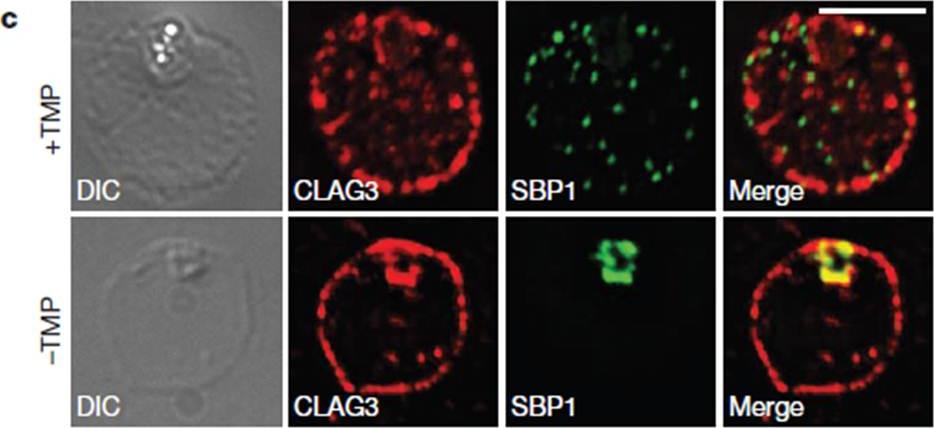
HSP101 is required for activation of PSAC but not trafficking of CLAG3 to the RBC periphery. CLAG3 proteins localize to the infected RBCperiphery and are the only parasite proteins known to be involved in PSAC activation IFA of ring-stage 13F10 parasites showing CLAG3 localization to the RBC membrane with or without TMP (DDD-stabilizing small molecule trimethoprim). TMP was removed in the late schizont stage and parasites were allowed to develop 18 h before fixation with 90% acetone/10% methanol. Scale bar, 5 mm. Beck JR, Muralidharan V, Oksman A, Goldberg DE. PTEX component HSP101 mediates export of diverse malaria effectors into host erythrocytes. Nature. 2014 Jul 16. [Epub ahead of print]
See original on MMP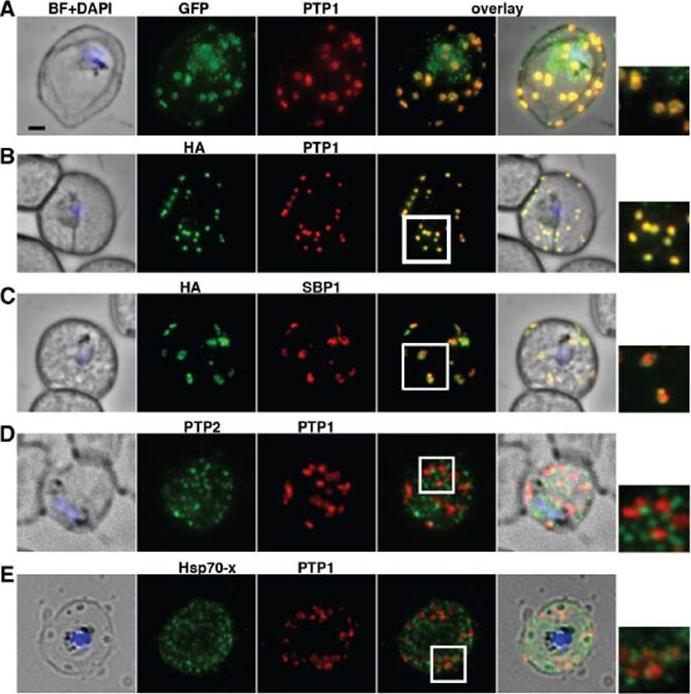
PfPTP1 is localised on Maurer’s clefts in P. falciparum-infected RBCs. (A) Immunofluorescence assay on CS2/PfPTP1-GFP cell line: the GFP signal (green) colocalises with that of the endogenous protein (PfPTP1; red); inset: enlargement of colocalisation pattern. (B) Immuno-fluorescence assay on CS2/PfPTP1-HA cell line: the HA signal (green) colocalises with the α-PfPTP1 signal (red); inset: enlargement of colocalisation pattern. (C) Immunofluorescence assay on CS2/PfPTP1-HA cell line: the HA-signal (green) colocalises partially with the MC resident protein SBP1 (red); inset: enlargement of colocalisation pattern. (D) Immunofluorescence assay on CS2 parental cell line: the PfPTP2-signal (green) does not overlap with the PfPTP1-signal (red); inset: enlargement of area where MC (PfPTP1) and electron dense vesicles (PfPTP2) are in close proximity. (E) Immunofluorescence assay on CS2 parental cell line: the PfHsp70-x-signal (green) colocalises partially with the PfPTP1- signal (red); inset: enlargement of partial colocalisation on J-Dots (PfHsp70-x). Scale bar: 1μm; same size of ROI in each image. Rug M, Cyrklaff M, Mikkonen A, Lemgruber L, Kuelzer S, Sanchez CP, Thompson J, Hanssen E, O'Neill M, Langer C, Lanzer M, Frischknecht F, Maier AG, Cowman AF. Export of virulence proteins by malaria-infected erythrocytes involves remodelling of host actin cytoskeleton. Blood. 2014 Aug 19 [Epub ahead of print]
See original on MMP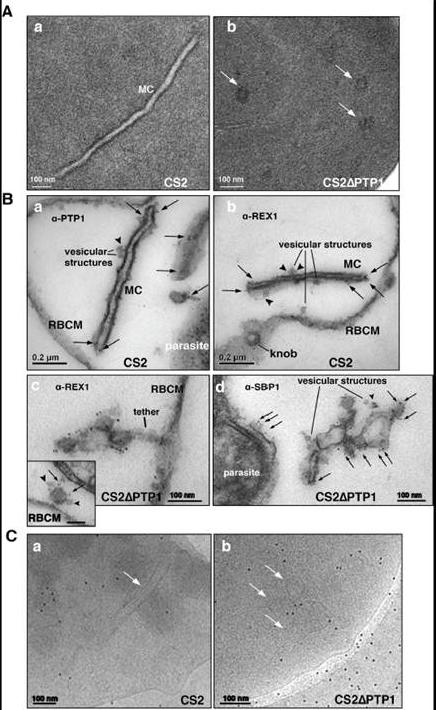
Comparison of transmission electron microscopical studies on CS2 versus CS2ΔPTP1 cell line.(A) Conventional chemical Fixation: a) CS2 parental line. MC (MC) display electron dense membranes and an electron lucent lumen; b) CS2ΔPTP1 cell line shows globular structures and no long lamellar MC structures (arrows). (B) Equinatoxin II treated cells with pre-embedding labelling: a) α-PfPTP1 antibodies or b) α-REX1 antibodies label the slender MC membranes (arrows) and vesicles (arrowheads) in the CS2 parasite line; c) α-REX1 or d) α-SBP1 antibodies label globular structures (arrows) and vesicular structures (arrowheads) in the CS2ΔPTP1 cell line. RBCM – red blood cell membrane. (C) The frozen hydrated samples show MC (arrow) in which the lumen looks similar to the RBC cytoplasm as described earlier. The CS2ΔPTP1 cell line shows aggregated globular structures (arrows). In CS2 typical single lamellae for MC were observed with an electron dense coat and translucent lumen (Aa). In contrast, CS2ΔPTP1 displayed electron dense areas randomly distributed through the RBC cytoplasm (Ab). The α-PfPTP1 antibodies line the MC lamella similar to REX1 localisation (B). Vesicular structures were observed that appeared to bud off MC and these had a dense lumen and less distinct membranes. These may be vesicles involved in trafficking between PVM and MC and/or MC to the RBC membrane (i.e. J-Dots). These structures were decorated withPfPTP1 and REX1 antibodies (Ba,b). In CS2ΔPTP1 the architecture of MC was disrupted showing large aggregated globular structures (Bc,d). SBP1 and REX1 were located on these structures. Single lamellae of MC were observed in CS2 (Ca) whereas globular structures were observed for CS2ΔPTP1 (Cb).Rug M, Cyrklaff M, Mikkonen A, Lemgruber L, Kuelzer S, Sanchez CP, Thompson J, Hanssen E, O'Neill M, Langer C, Lanzer M, Frischknecht F, Maier AG, Cowman AF. Export of virulence proteins by malaria-infected erythrocytes involves remodelling of host actin cytoskeleton. Blood. 2014 Aug 19 [Epub ahead of print]
See original on MMP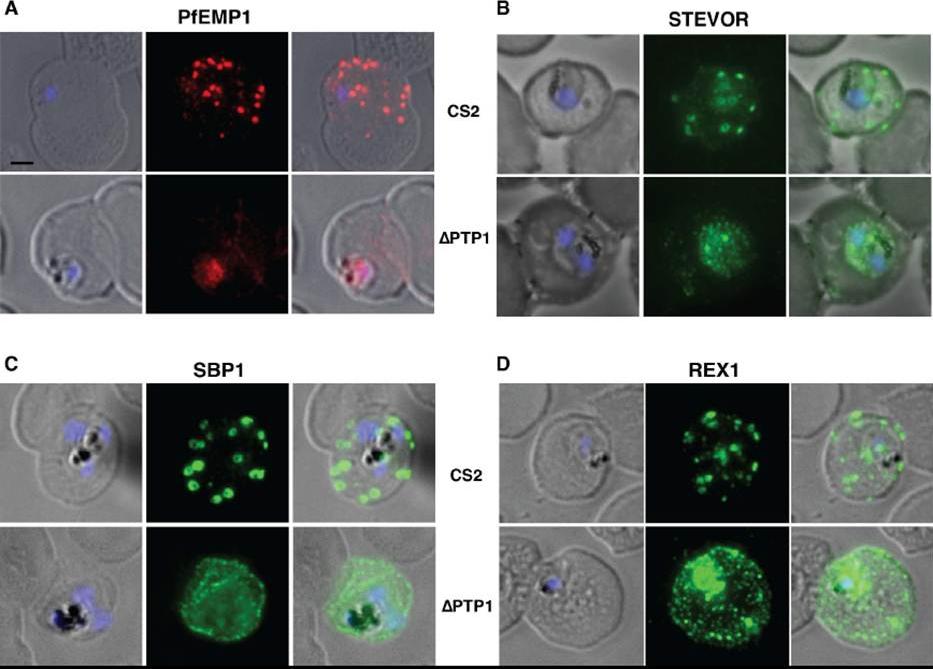
Immunofluorescence assay on CS2 (upper panels) versus CS2ΔPTP1 parasites (lower panels). Immunofluorescence assay probed with a-PfEMP1 antibody (A), a-STEVOR antibody (B), a -SBP 1 antibody (C) and a -REX 1 antibody (D). Scale bar: 1 μm; same size of ROI in each image. In CS2ΔPTP1 the architecture of MC was disrupted showing large aggregated globular structures. SBP1 and REX1 were located on these structures reflecting the results obtained by IFA.Rug M, Cyrklaff M, Mikkonen A, Lemgruber L, Kuelzer S, Sanchez CP, Thompson J, Hanssen E, O'Neill M, Langer C, Lanzer M, Frischknecht F, Maier AG, Cowman AF. Export of virulence proteins by malaria-infected erythrocytes involves remodelling of host actin cytoskeleton. Blood. 2014 Aug 19 [Epub ahead of print]
See original on MMP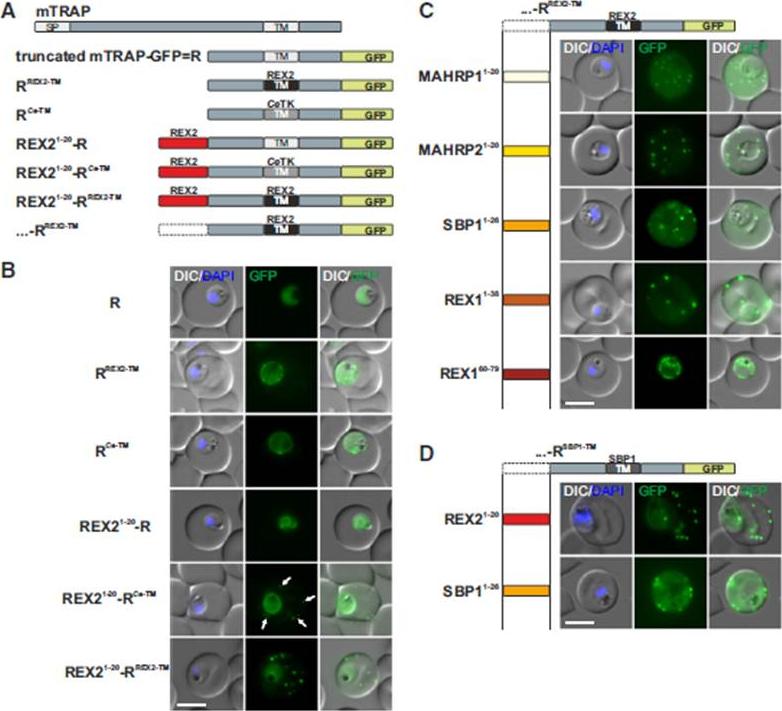
N Termini of PNEPs Are Sufficient for Export of RREX2-TM, (A) Schematic of mTRAP fusion constructs. (B) Representative images of live P. falciparum parasites expressing the constructs shown in (A). DIC, differential interference contrast; nuclei were stained with DAPI. Arrows indicate limited staining reminiscent of Maurer’s clefts. (C and D) Images of live P. falciparum parasites expressing RREX2-TM (C) and RSBP1-TM (D) fused with the PNEP N termini indicated. Panels are as in (B). Size bars represent 5 mm. The mTRAP backbone, different TMs, and the SP (signal peptide) are shown in different shades of gray; N-terminally appended regions of PNEPs are shown in shades from yellow to dark red. Ce-TM, C. elegans TK TM. The unmodified truncated mTRAP fused to GFP (‘‘R’’ for ‘‘reporter’’) does not contain any export-relevant sequences. This protein was found evenly distributed in the parasite cytosol (B). When the mTRAP TM in R was replaced with that of the PNEP REX2 or a previously used heterologous TM of a C.elegans tyrosine kinase (TK) (a TM that does not promote export of REX2 and does not change topology;), the R then entered the secretory pathway and was found in the parasite periphery (PPM, PV, or PVM) but was not exported.Grüring C, Heiber A, Kruse F, Flemming S, Franci G, Colombo SF, Fasana E,Schoeler H, Borgese N, Stunnenberg HG, Przyborski JM, Gilberger TW, Spielmann T. Uncovering common principles in protein export of malaria parasites. Cell Host Microbe. 2012 12(5):717-29.
See original on MMP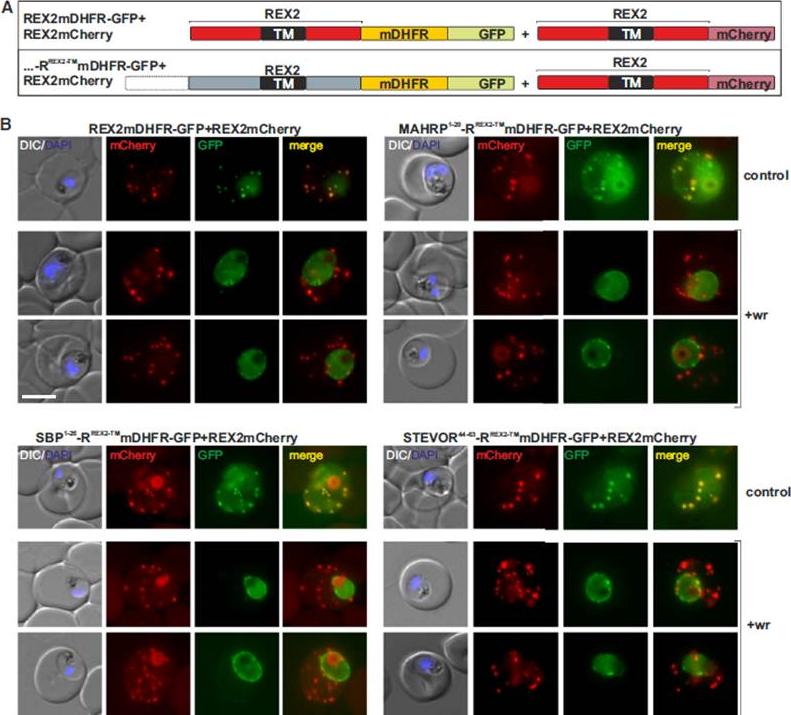
Blocking Unfolding Arrests Export of PNEPs. (A) Schematic of the constructs of the cell lines shown in (B). (B) Parasites expressing REX2mCherry (red) together with the mDHFR-GFP-tagged constructs (green) indicated above each panel. Two images are shown per cell line in the WR99210 (+wr)-treated samples to demonstrate that in different cells the parasite peripheral staining of the blocked protein displayed either a smooth or a more focal pattern. Merge, overlay of the red and green signals. The size bar represents 5 mm.Grüring C, Heiber A, Kruse F, Flemming S, Franci G, Colombo SF, Fasana E, Schoeler H, Borgese N, Stunnenberg HG, Przyborski JM, Gilberger TW, Spielmann T. Uncovering common principles in protein export of malaria parasites. Cell Host Microbe. 2012 12(5):717-29.
See original on MMP
Upper panel: PFD0495c is exported to the erythrocyte. (iii) fluorescence image of pfd0495c-gfp expressing parasites. Fluorescence image of pfd0495c-gfp expressing parasites (iv) fluorescence and DIC image showing export ofPDF0495c chimera to the erythrocyte (e) periphery (arrow) andintraerythrocytic spots (arrowhead).Lower panel: PFD0495c is found within intraerythrocytic structures. 3D7 early schizonts were fixed and probed with antibodies to PFD0495c (i) and Skeleton Binding Protein (SBP1; ii) for immunofluorescence. Endogenous PFD0495c is found associated with the parasite and within intraerythrocytic structures, vesicular (arrowhead) and tubular (arrow) structures, distinct from Maurer’s Clefts (iii).Tamez PA, Bhattacharjee S, van Ooij C, Hiller NL, Llinás M, Balu B, Adams JH, Haldar K. An erythrocyte vesicle protein exported by the malaria parasite promotes tubovesicular lipid import from the host cell surface. PLoS Pathog. 2008 Aug 8;4(8):e1000118.
See original on MMP
Kinetics of export of PfSBP1CAD in various P. falciparum-infected erythrocytes. P. falciparum-infected erythrocytes were taken from culture at the time points indicated after the addition of the anti-aggregation ligand and immediately analyzed by live cell confocal fluorescence microscopy. Tightly synchronized cultures at the trophozoite-stage (18–22 h post invasion) were used.Kilian N, Srismith S, Dittmer M, Ouermi D, Bisseye C, Simpore J, Cyrklaff M, Sanchez CP, Lanzer M. Hemoglobin S and C affect protein export in Plasmodium falciparum-infected erythrocytes. Biol Open. 2015 4(3):400-10.
See original on MMP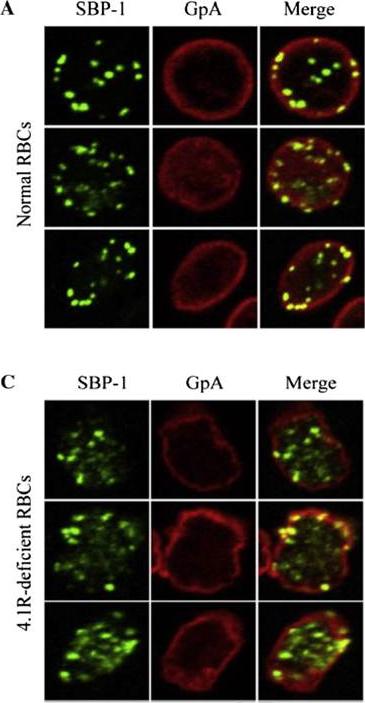
Localisation of SBP-1 in normal and protein 4.1R-deficient red blood cells. Representative confocal immunofluorescence images of normal (A) and 4.1R-deficient (C) RBCs immunolabelled for Maurer's clefts (SBP1; green) and the RBCmembrane skeleton (Glycophorin A, GpA; red). This data strongly supports that the interaction between SBP1-C and protein 4.1R plays a significant role in localising Maurer's clefts to their final destination in the PRBC, tethered to the RBC membrane skeleton.Kats LM, Proellocks NI, Buckingham DW, Blanc L, Hale J, Guo X, Pei X, Herrmann S, Hanssen EG, Coppel RL, Mohandas N, An X, Cooke BM. Interactions between Plasmodium falciparum skeleton-binding protein 1 and the membrane skeleton of malaria-infected red blood cells. Biochim Biophys Acta. 2015 Apr 1848(7):1619-1628. PMID:
See original on MMP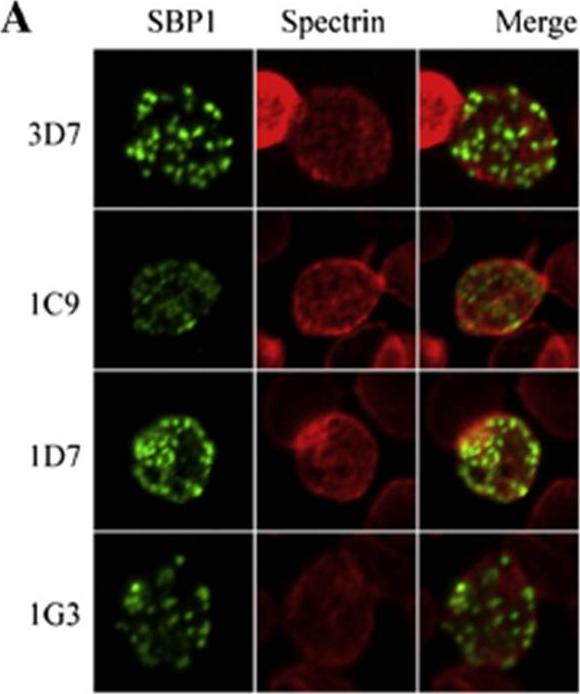
Functional analysis of the SBP1 C-terminal domain. A. Representative images from immunofluorescence analysis RBCs infected with wild type 3D7 parasites or the three clonal parasite lines for the SBP1–AMA1-C chimeras (clones 1C9, 1D7 and 1G3 expressing chimeric SBP1 comprising the N-terminal domain of SBP1 and the C-terminal domain of AMA1 (SBP1–AMA1-C). Green represents SBP1 and red is spectrin. Distribution of the chimeras in PRBCs was not significantly different to that found in wild type, parental 3D7 parasites.Kats LM, Proellocks NI, Buckingham DW, Blanc L, Hale J, Guo X, Pei X, Herrmann S, Hanssen EG, Coppel RL, Mohandas N, An X, Cooke BM. Interactions between Plasmodium falciparum skeleton-binding protein 1 and the membrane skeleton of malaria-infected red blood cells. Biochim Biophys Acta. 2015 Apr 1848(7):1619-1628.
See original on MMP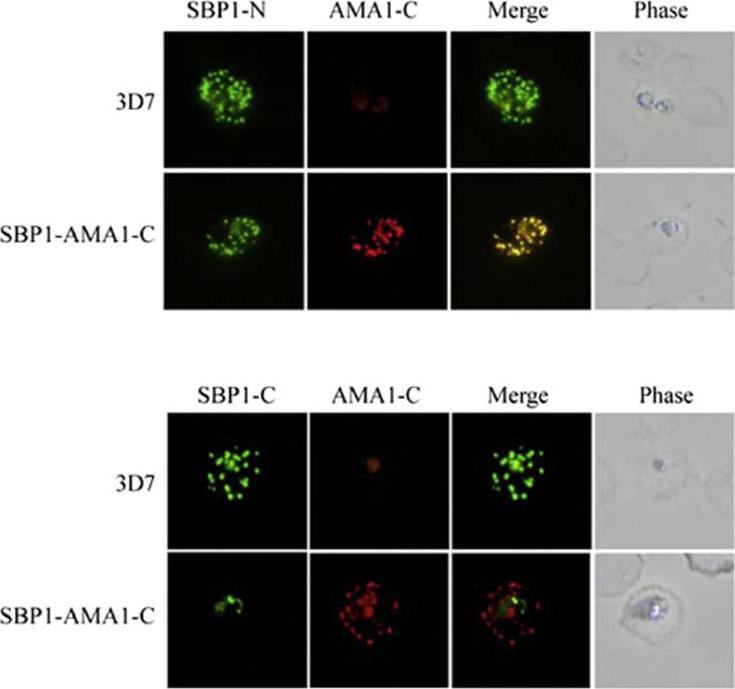
Localisation of SBP1 C-terminal mutants. Immunofluorescence analysis of RBCs infected with either normal (3D7) or transgenic parasites expressing a chimeric SBP1 protein in which the C-terminal domain of SBP1 was replaced with the C-terminal domain of P. falciparum AMA1 (SBP1–AMA1-C). Chimeras localised this protein correctly to Murer’s Clefts. Kats LM, Proellocks NI, Buckingham DW, Blanc L, Hale J, Guo X, Pei X, Herrmann S, Hanssen EG, Coppel RL, Mohandas N, An X, Cooke BM. Interactions between Plasmodium falciparum skeleton-binding protein 1 and the membrane skeleton of malaria-infected red blood cells. Biochim Biophys Acta. 2015 1848(7):1619-1628.
See original on MMP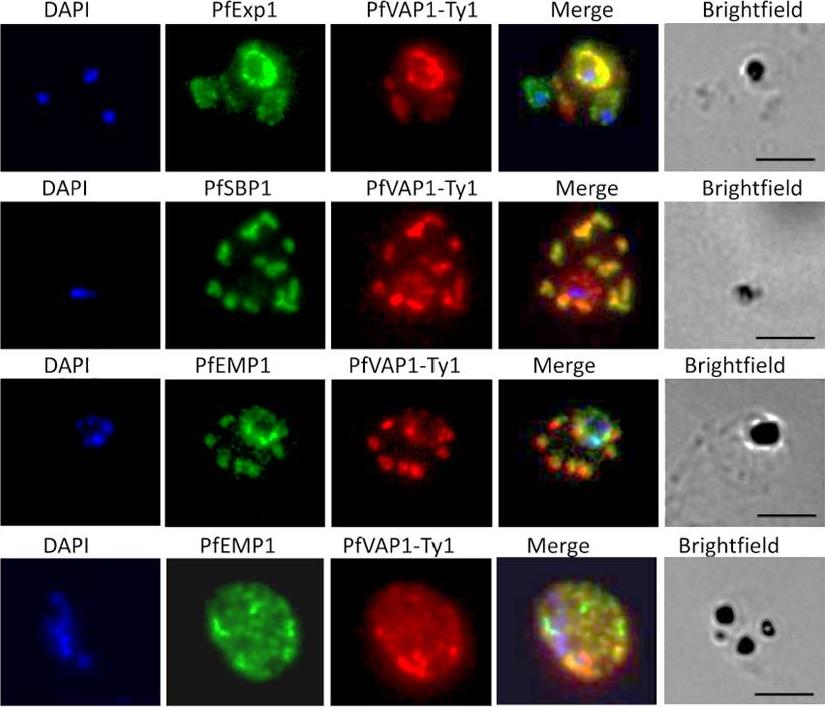
Localisation of PfVAP1 in complemented D10 parasites. Ty1-tagged D10 Pfvap1 parasites were labelled with anti-Ty1 monoclonal antibody to determine the localisation of the protein. In early trophozoites PfVAP1 is associated with the parasitophorous vacuole (PV) as determined by double labelling with PfEXP1. It is then trafficked via the Maurer’s clefts (PfSBP1) to the infected erythrocyte membrane. In the Maurer’s clefts, PfVAP1 co-localises with PfEMP1 (3rd row) and appears to localise in distinct regions, separate from PfEMP1 at the erythrocyte membrane (4th row). Scale bars = 5 μm.Nacer A, Claes A, Roberts A, Scheidig-Benatar C, Sakamoto H, Ghorbal M, Lopez-Rubio JJ, Mattei D. Discovery of a novel and conserved Plasmodium falciparum exported protein that is important for adhesion of PfEMP1 at the surface of infected erythrocytes. Cell Microbiol. 2015 Feb 23
See original on MMP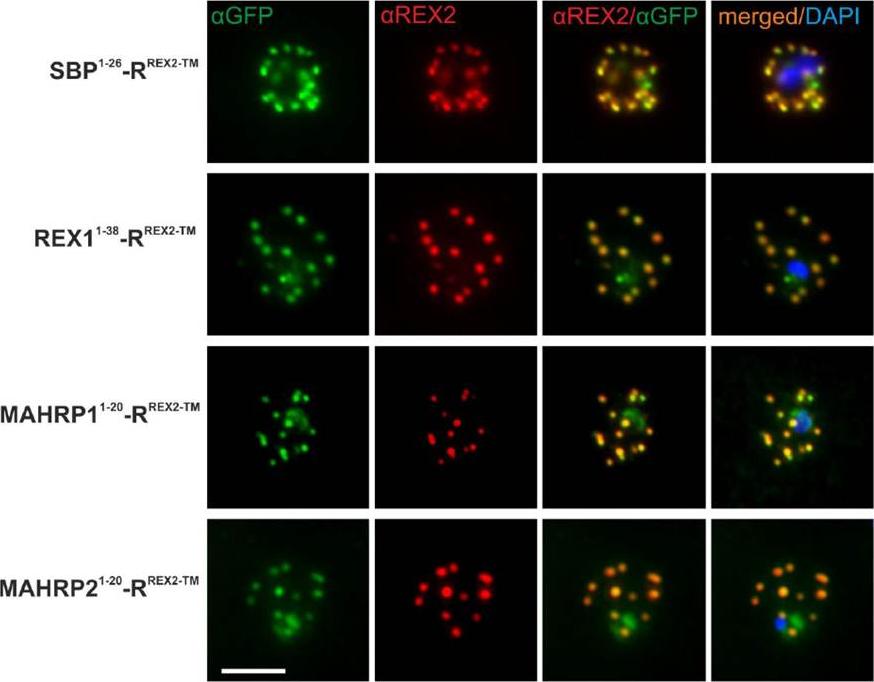
Immunofluorescence Assays Confirm Maurer’s Clefts Localization of PNEP N Termini mTRAP Reporter Fusions. Acetone fixed parasites expressing the reporter indicated on the left were probed with anti-GFP and a serum reacting with the C-terminus of REX2. Under these fixing conditions soluble proteins in the host cell are lost, leading to absence of the soluble pool of the reporter. DIC, differential interference contrast; DAPI, nuclei. Size bar: 5m.Grüring C, Heiber A, Kruse F, Ungefehr J, Gilberger TW, Spielmann T. Development and host cell modifications of Plasmodium falciparum blood stages in four dimensions. Nat Commun. 2011 2:165. PMID:
See original on MMP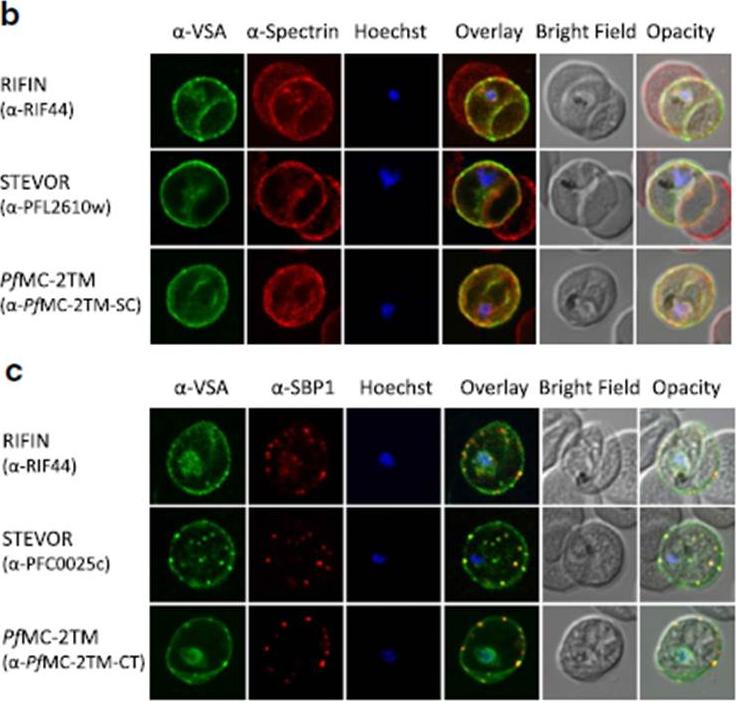
b Co-localization of α-RIF44, STEVOR α-PFL2610w and α-PfMC-2TM-SC (green) with human spectrin (red). c Co-localization of α-RIF44, STEVOR α-PFC0025c and α-PfMC-2TM-CT (green) with SBP1 (red).Bachmann A, Scholz JA, Janßen M, Klinkert MQ, Tannich E, Bruchhaus I, Petter M. A comparative study of the localization and membrane topology of members of the RIFIN, STEVOR and PfMC-2TM protein families in Plasmodium falciparum-infected erythrocytes. Malar J. 2015 Jul 14:274.
See original on MMP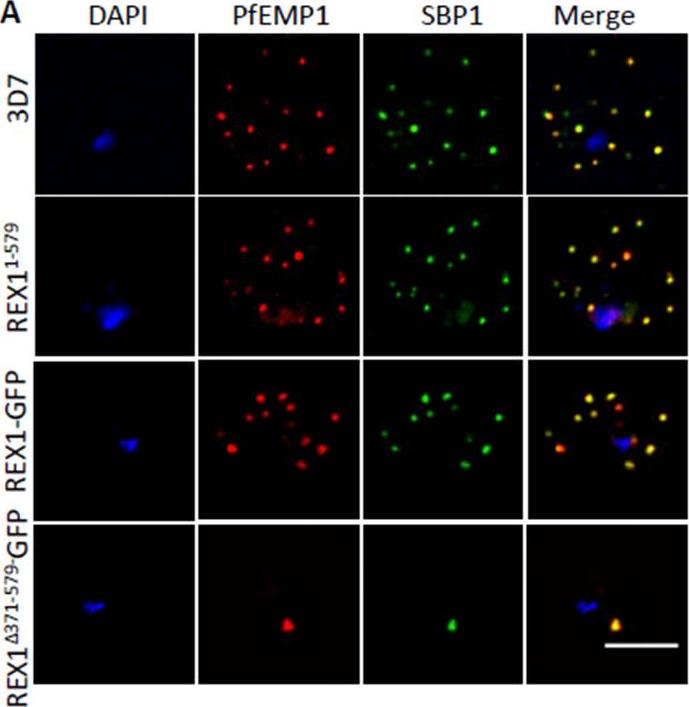
PfEMP1 surface-exposure and cytoadherence of REX1 transfectants. A. Immunofluorescence microscopy of acetone-fixed RBCs infected with 3D7 and REX1 transfectants. Maurer’s clefts were identified by immunolabeling with anti-SBP1 (green). Anti-PfEMP1 antibodies (red) revealed colocation of the virulence protein with Maurer’s clefts. The nuclei are stained with DAPI (blue). Scale bar = 3 μm. Accumulation of PfEMP1 at the Maurer’s clefts in the 3D7 wildtype parasites, was observed as confirmed by co-labelling with the Maurer’s cleft marker, SBP1. PfEMP1 is also delivered to the Maurer's clefts in the REX11-579, REX1-GFP and REX1Δ371-579-GFP mutant parasite lines. Thus despite the aberrant morphology there is no obvious defect in trafficking of PfEMP1 from the parasite to the stacked Maurer’s clefts.McHugh E, Batinovic S, Hanssen E, McMillan PJ, Kenny S, Griffin MD, Crawford S, Trenholme KR, Gardiner DL, Dixon MW, Tilley L. A repeat sequence domain of the ring-exported protein-1 of Plasmodium falciparum controls export machinery architecture and virulence protein trafficking. Mol Microbiol. 2015 Aug 24. [Epub ahead of print]
See original on MMP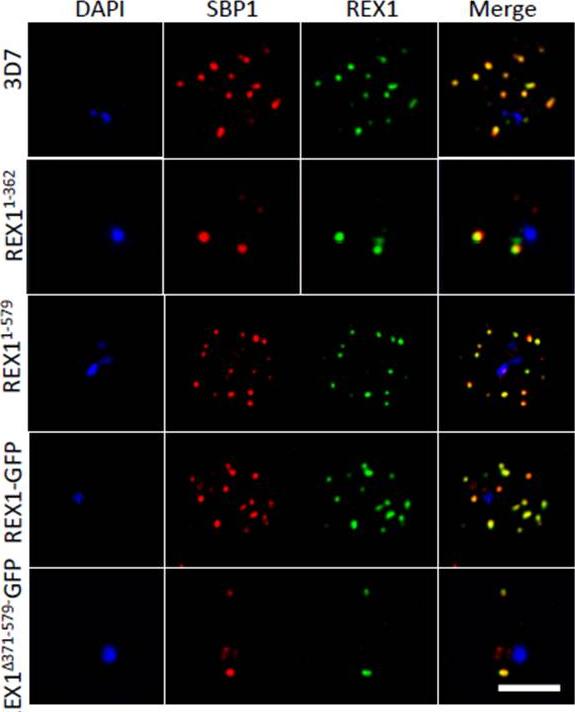
Deletion of the repeat region of REX1 decreases the number of Maurer's cleft puncta. A. Immunofluorescence microscopy of acetone-fixed infected RBCs probed with anti-SBP1 (red) and anti-REX1 (green). Nuclei are stained with DAPI (blue). Scale bar = 3 μm. Full-length REX1-GFP is correctly localized at the Maurer's clefts and that these transfectants exhibit an average number of puncta equivalent to that of the 3D7 parent. By contrast, the REX1Δ371-579-GFP parasites exhibited a striking Maurer’s cleft phenotype, with only 2 + 0.3 punctate structures observed in the infected RBC cytoplasm.McHugh E, Batinovic S, Hanssen E, McMillan PJ, Kenny S, Griffin MD, Crawford S, Trenholme KR, Gardiner DL, Dixon MW, Tilley L. A repeat sequence domain of the ring-exported protein-1 of Plasmodium falciparum controls export machinery architecture and virulence protein trafficking. Mol Microbiol. 2015 Aug 24. [Epub ahead of print]
See original on MMP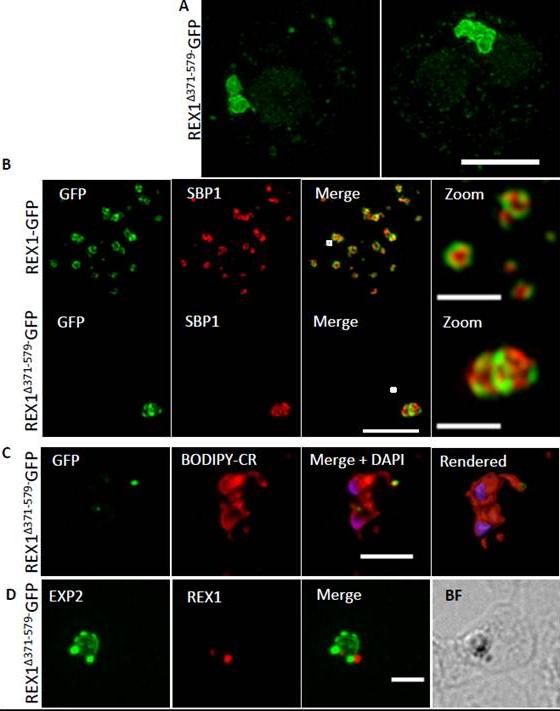
REX1(Δ371–579)-GFP parasites exhibit giant Maurer’s clefts. A. REX1(Δ371–579)-GFPinfected RBCs fixed with paraformaldehyde, permeabilized with EqtII and labelled with anti-GFP. B. REX1-GFP and REX1(Δ371–579)-GFP-infected RBCs were fixed with paraformaldehyde/glutaraldehyde and probed with anti-GFP (green) and anti-SBP1 (red). Samples were examined using 3D-SIM. 3D-SIM reveals giant Maurer's clefts with an apparently convoluted surfaceC. REX1(Δ371–579)-GFP-infected RBCs were labelled with BODIPY-ceramide, fixed with paraformaldehyde/ glutaraldehyde, labelled with DAPI and imaged using widefield deconvolution microscopy. The right hand panel shows rendering of the surface of the 3D structure using Imaris software. REX1Δ371-579-GFP appears to accumulate at discrete regions of the PVM as does EXP-2. D. REX1(Δ371–579)-GFP-infected RBCs were fixed with paraformaldehyde/ glutaraldehyde and probed with anti-EXP2 (green) and anti-REX1 (red). Samples were examined using deconvolution microscopy. Scale bars = 3 μm; zoom bar = 1 μm.McHugh E, Batinovic S, Hanssen E, McMillan PJ, Kenny S, Griffin MD, Crawford S, Trenholme KR, Gardiner DL, Dixon MW, Tilley L. A repeat sequence domain of the ring-exported protein-1 of Plasmodium falciparum controls export machinery architecture and virulence protein trafficking. Mol Microbiol. 2015 Aug 24. [Epub ahead of print] PMID:
See original on MMP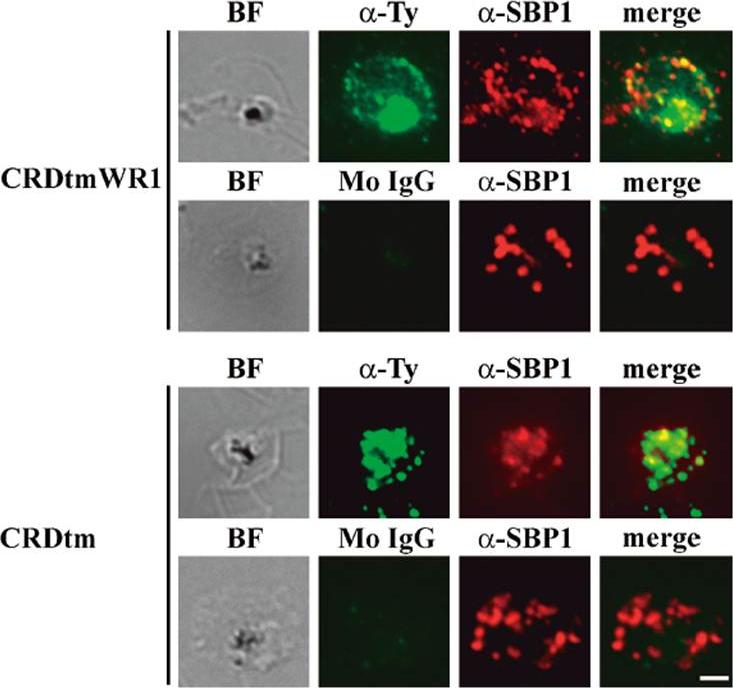
Indirect immunofluorescence assay of recombinant CRDtmWR1 (recombinant SURFIN4.2 proteins containing intracellular region) and CRDtm (recombinant SURFIN4.2 proteins not containing intracellular region). CRDtmWR1 and CRDtm were dual stained with mouse anti-Ty antibody (α-Ty, green; Monoclonal antibody raised in mouse against the Ty1 tag (amino acid sequence EVHTNQDPLD) for recombinant SURFIN4.2 and rabbit anti-PfSBP1 serum (α-SBP1, red) for Maurer’s clefts. Dual staining negative control images with mouse normal IgG (Mo IgG) and anti-PfSBP1 are also shown. Bright field images (BF) are shown, as well as merged images (merge) of α-Ty, α-SBP1, and DAPI (blue) DNA staining. The bar = 2 μm.Kagaya W, Miyazaki S, Yahata K, Ohta N, Kaneko O. The Cytoplasmic Region of Plasmodium falciparum SURFIN4.2 Is Required for Transport from Maurer's Clefts to the Red Blood Cell Surface. Trop Med Health. 2015 Dec;43(4):265-72.
See original on MMP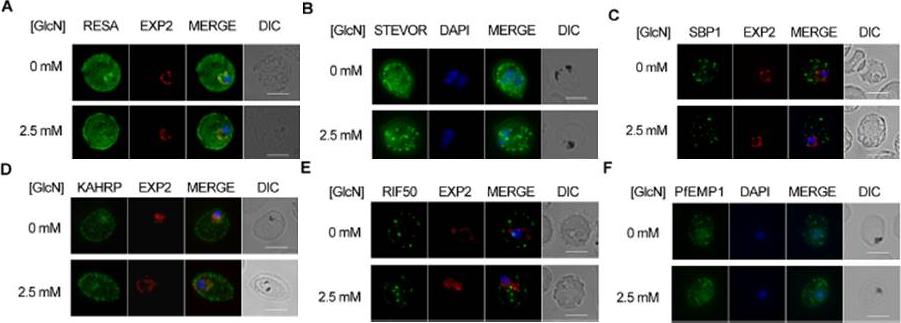
Effect of PTEX88 knockdown on P. falciparum protein export. Immunofluorescence assays showing that exported proteins A: RESA, B: STEVOR, C: SBP1, D: KAHRP, E: RIF50, F: PfEMP1 are still exported after knockdown of PTEX88. RESA (an early expressed PEXEL protein), STEVOR (a PEXEL protein containing transmembrane domains), skeleton binding protein 1 (SBP1; a PNEP), KAHRP (a soluble PEXEL protein), RIF50 (a PEXEL protein containing transmembrane domains) and PfEMP1 (a PNEP containing a transmembrane domain that is exported to the erythrocyte surface.Chisholm SA, McHugh E, Lundie R, Dixon MW, Ghosh S, O'Keefe M, Tilley L, Kalanon M, de Koning-Ward TF. Contrasting Inducible Knockdown of the Auxiliary PTEX Component PTEX88 in P. falciparum and P. berghei Unmasks a Role in Parasite Virulence. PLoS One. 2016 Feb 17;11(2):e0149296.
See original on MMP
The distance between blocking domain and TM is important for co-blocking and BPTI sensitivity. (A-E) Representative images of live P. falciparum parasites expressing the constructs shown schematically above each panel (numbers refer to the length of the amino acids sequence between TM and blocking domain). (A-C) Parasites grown in the presence (+WR) or absence of WR (control). DIC, differential interference contrast. Size bars: 5 μmMesén-Ramírez P, Reinsch F, Blancke Soares A, Bergmann B, Ullrich AK, Tenzer S, Spielmann T. Stable Translocation Intermediates Jam Global Protein Export in Plasmodium falciparum Parasites and Link the PTEX Component EXP2 with Translocation Activity. PLoS Pathog. 2016 May 11;12(5):e1005618. “
See original on MMP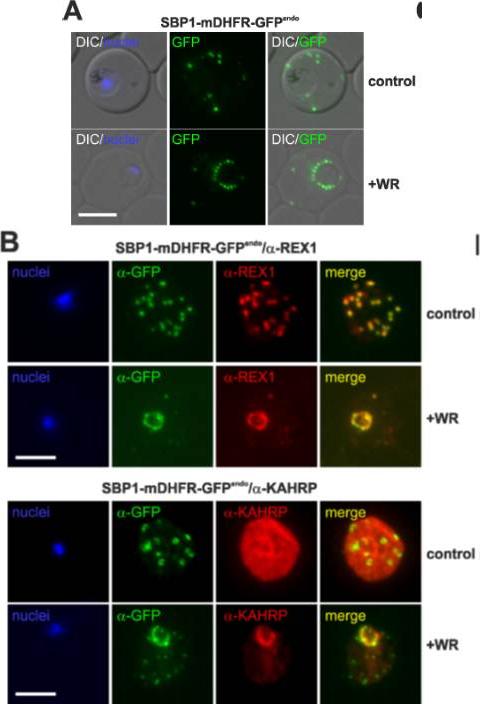
Translocation arrested substrates induce a pan export block that slows parasite development. (A) Representative live fluorescence images of the cell line expressing endogenous SBP1 fused to mDHFR-GFP (SBP1-mDHFR-GFPendo) grown with (+WR) and without WR (control). DIC, differential interference contrast. Size bar: 5 μm. (B) IFA of SBP1-mDHFR-GFPendo parasites using α-REX1 and α-KAHRP in parasites treated with (+WR) and without (control) WR. Size bars: 5 μm. Mesén-Ramírez P, Reinsch F, Blancke Soares A, Bergmann B, Ullrich AK, Tenzer S, Spielmann T. Stable Translocation Intermediates Jam Global Protein Export in Plasmodium falciparum Parasites and Link the PTEX Component EXP2 with Translocation Activity. PLoS Pathog. 2016 May 11;12(5):e1005618.
See original on MMP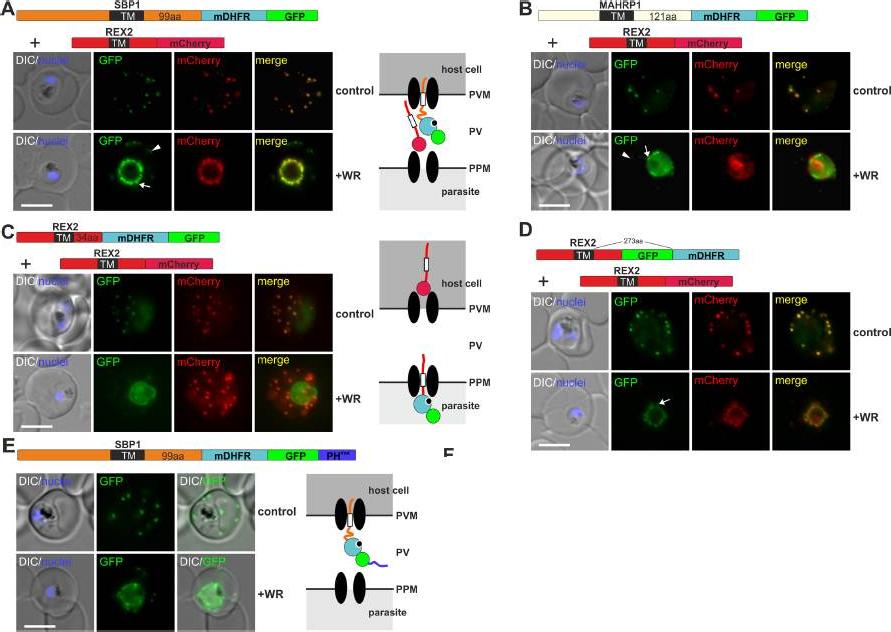
Mouse DHFR fusions can clog the PVM translocon and co-block the export of other proteins. (A-E) Representative images of live P. falciparum parasites grown in the presence (+WR) or absence of WR (control) and expressing the constructs shown schematically above each panel. DIC, differential interference contrast. Arrowheads indicate faint signals at the Maurer’s clefts; arrows show mobile protrusions (note that they do not overlap in the red and the green signal due to movement between capture of the images). Size bars: 5 μm. Schematics to the right show the location of the fusion protein containing the folded WR-bound mDHFR domain (light blue circle with smaller black circle in binding pocket) and the co-expressed REX2 (red line) fused to mCherry (red circle); white box, TM; green circle, GFP; blue line, protease sensitive mutated PH domain.Mesén-Ramírez P, Reinsch F, Blancke Soares A, Bergmann B, Ullrich AK, Tenzer S, Spielmann T. Stable Translocation Intermediates Jam Global Protein Export in Plasmodium falciparum Parasites and Link the PTEX Component EXP2 withTranslocation Activity. PLoS Pathog. 2016 May 11;12(5):e1005618.
See original on MMP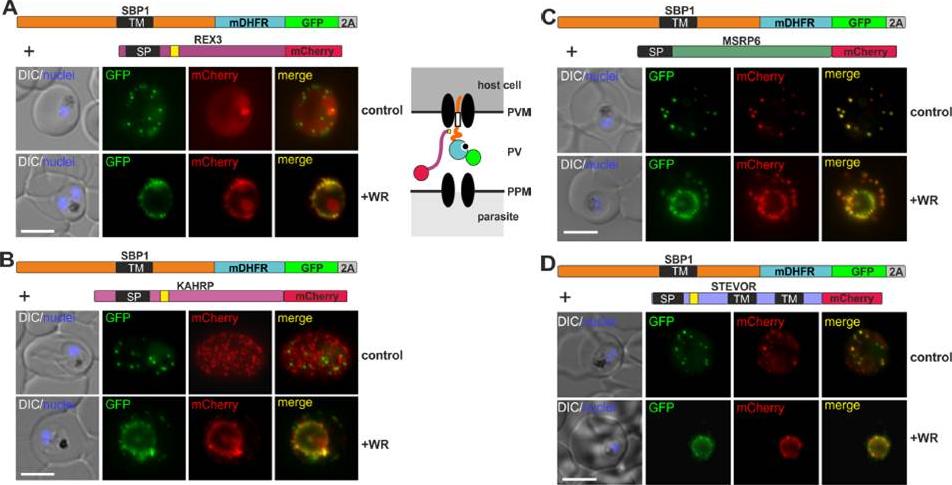
Different types of proteins pass through the same translocon. (A-D) Representative images of live P. falciparum parasites grown in the presence (+WR) or absence of WR (control), expressing the constructs shown schematically above each panel. The skip peptide is indicated by a grey box labelled 2A. The two proteins expressed from the same open reading frame are shown skipped. DIC, differential interference contrast. Size bars: 5 μm. A schematic of the co-block is shown for A. The yellow box indicates the mature PEXEL.Mesén-Ramírez P, Reinsch F, Blancke Soares A, Bergmann B, Ullrich AK, Tenzer S, Spielmann T. Stable Translocation Intermediates Jam Global Protein Export in Plasmodium falciparum Parasites and Link the PTEX Component EXP2 with Translocation Activity. PLoS Pathog. 2016 May 11;12(5):e1005618.
See original on MMP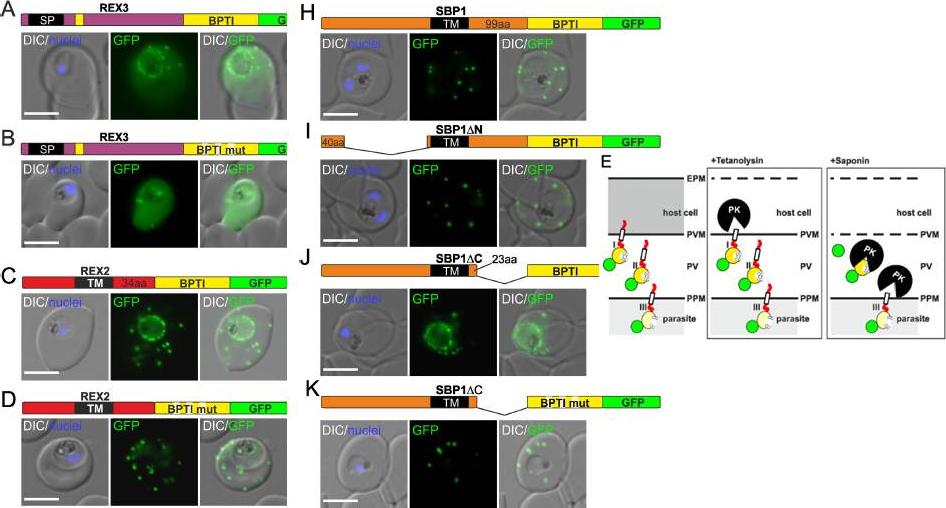
Fusion with the redox sensitive BPTI reveals a second translocation step for TM proteins. (A-D and F-L) Representative images of live P. falciparum parasites expressing the constructs shown schematically above each panel. Hydrophobic regions (SP, signal peptide; TM, transmembrane domain) are in black, the PEXEL motif in yellow. Numbers refer to amino acids (aa). Red boxes labelled C, additional REX2 C-termini. Interrupted yellow box, mutated BPTI (BPTImut). DIC, differential interference contrast. Size bars: 5 μm. (E) Schematic for the protease K (PK) protection assay. Left, intact infected RBC with 3 possibilities (I, II, III) for the location of the fusion construct: I, protein is integral to PVM; II, protein is freely accessible in the PV; III, protein is integral to PPM. Middle, after permeabilisation of the erythrocyte plasma membrane (EPM) with tetanolysin the N-terminus of the construct will be digested if it is in the PVM (I), but remains intact in situation II and III. Right, after permeabilisation of the PVM with saponin, the constructs will be digested if it is in the PVM (I) or the PV (II) but if in the PPM (III), an N-terminally truncated fragment will be generated. Red, exported protein; white box, TM; yellow, BPTI with double cysteine bonds; green, GFP. Mesén-Ramírez P, Reinsch F, Blancke Soares A, Bergmann B, Ullrich AK, Tenzer S, Spielmann T. Stable Translocation Intermediates Jam Global Protein Export in Plasmodium falciparum Parasites and Link the PTEX Component EXP2 withTranslocation Activity. PLoS Pathog. 2016 May 11;12(5):e1005618.
See original on MMP
Immunolocalization of c-myc epitope-tagged PFE1605w PHIST protein within infected erythrocytes. A) Synchronized parasites were harvested at different time points after Percoll-sorbitol purification, air-dried and fixed with ice-cold methanol. Epitope-tagged proteins were stained with anti-c-myc monoclonal antibodies that were conjugated with FITC. Nuclei were stained with DAPI (blue). The abbreviations used to describe parasite stages are as follows: 22 h, late ring stage; 30 h, early-trophozoite stage; 42 h, mid-trophozoite stage; 48 h, late trophozoite stage; and schizont stage. B) Co-localization was assayed using rabbit polyclonal SBP1 followed by goat anti-rabbit IgG conjugated with Alexa 594 (red). C) The pattern of expression of c-myc epitope tagged PFE1605w PHIST protein is dependent on the fixation protocol. When infected erythrocytes are fixed in solution with 1% paraformaldehyde, 0.075% glutaraldehyde, then PHIST protein shows a broader punctate expression than the Maurer’s cleft marker, SPB1. Moreira CK, Naissant B, Coppi A, Bennett BL, Aime E, Franke-Fayard B, Janse CJ, Coppens I, Sinnis P, Templeton TJ. The Plasmodium PHIST and RESA-Like Protein Families of Human and Rodent Malaria Parasites. PLoS One. 2016 Mar 29;11(3):e0152510.
See original on MMP
The export of KAHRP and SBP1 in the PTEX150Δ125-HA mutant compared to full length PTEX150-HA parasites. PTEX150-HA and PTEX150Δ125-HA parasites were synchronized to an invasion window of 5 h. The regions occupied by the parasite are indicated by DAPI and EXP2 staining. KAHRP is a PEXEL protein, which initially displays a diffuse staining of the erythrocyte cytosol before accumulating in punctate regions below the plasma membrane of the host cell. SBP is a PNEP that localizes to punctate vesicular structures called Maurer’s clefts (MC) in the host cytosol. IFAs were counterstained with antibodies to EXP2 to delineate where the boundaries of the parasite. No significant difference between the PTEX150Δ125-HA and PTEX150-HA parasite lines was observed.Elsworth B, Sanders PR, Nebl T, Batinovic S, Kalanon M, Nie CQ, Charnaud SC, Bullen HE, de Koning Ward TF, Tilley L, Crabb BS, Gilson PR. Proteomic analysis reveals novel proteins associated with the Plasmodium protein exporter PTEX and a loss of complex stability upon truncation of the core PTEX component, PTEX150. Cell Microbiol. 2016 18(11):1551-1569.
See original on MMP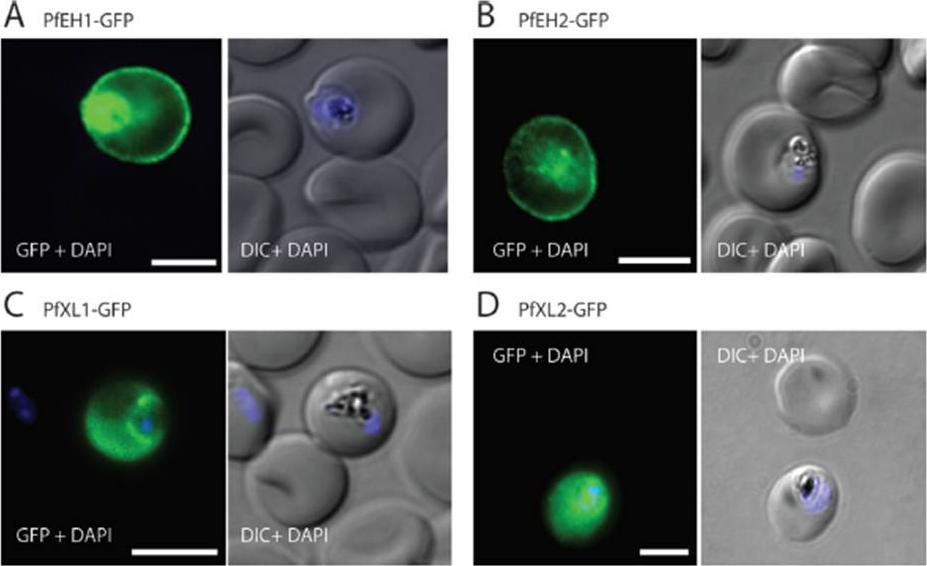
The export of KAHRP and SBP1 in the PTEX150Δ125-HA mutant compared to full length PTEX150-HA parasites. PTEX150-HA and PTEX150Δ125-HA parasites were synchronized to an invasion window of 5 h. The regions occupied by the parasite are indicated by DAPI and EXP2 staining. KAHRP is a PEXEL protein, which initially displays a diffuse staining of the erythrocyte cytosol before accumulating in punctate regions below the plasma membrane of the host cell. SBP is a PNEP that localizes to punctate vesicular structures called Maurer’s clefts (MC) in the host cytosol. IFAs were counterstained with antibodies to EXP2 to delineate where the boundaries of the parasite. No significant difference between the PTEX150Δ125-HA and PTEX150-HA parasite lines was observed.Elsworth B, Sanders PR, Nebl T, Batinovic S, Kalanon M, Nie CQ, Charnaud SC, Bullen HE, de Koning Ward TF, Tilley L, Crabb BS, Gilson PR. Proteomic analysis reveals novel proteins associated with the Plasmodium protein exporter PTEX and a loss of complex stability upon truncation of the core PTEX component, PTEX150. Cell Microbiol. 2016 18(11):1551-1569.
See original on MMP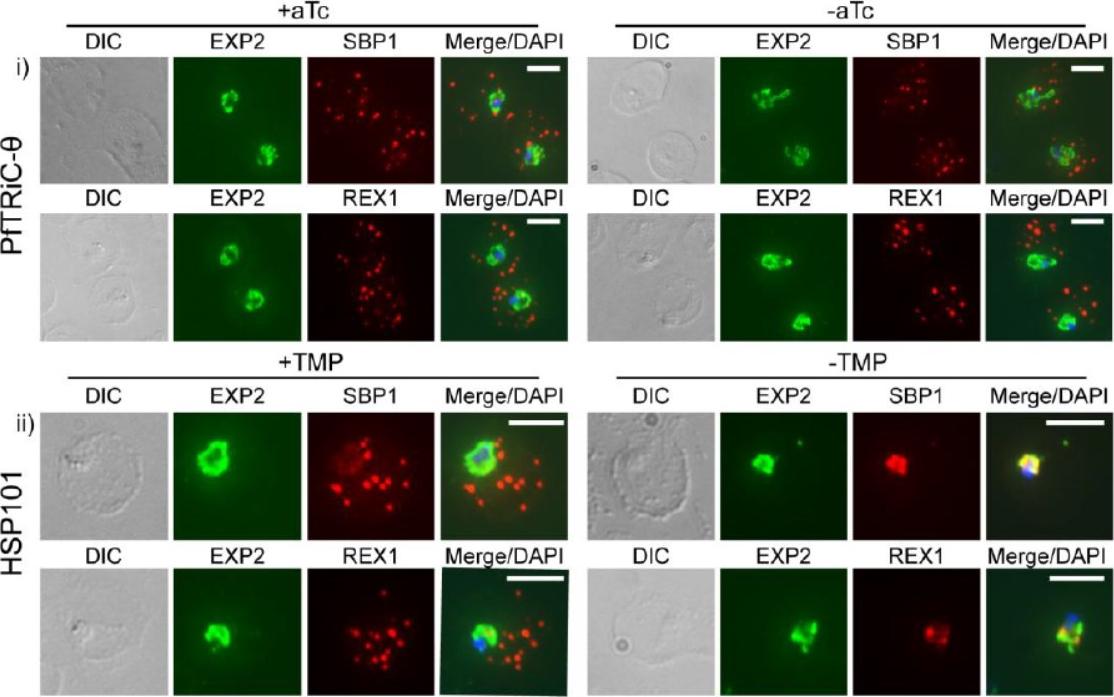
Loss of PfTRiC-θ does not alter export of PfSBP1 or PfREX1. IFA of acetone-fixed ring-stage infected RBC. i) PfTRiC-θ ± 0.5 μM aTc (anhydrotetracycline), ii) HSP101 ± 10 μM trimethoprim (TMP). DAPI stains the parasite nuclei and PfEXP2 delineates the parasite parasitophorous vacuole compartment. Scale bars = 5 μm. Data are representative of two independent assays. DIC = differential interference contrast. Loss of the PfTRiC complex does not alter export of PfSBP1 or PfREX1 (i). This was in contrast to the block in export of PfSBP1 and PfREX1 observed upon TMP removal in the previously characterized PfHSP101-DDD line (ii).Spillman NJ, Beck JR, Ganesan SM, Niles JC, Goldberg DE. The chaperonin TRiC forms an oligomeric complex in the malaria parasite cytosol. Cell Microbiol. 2017 Jan 9.
See original on MMP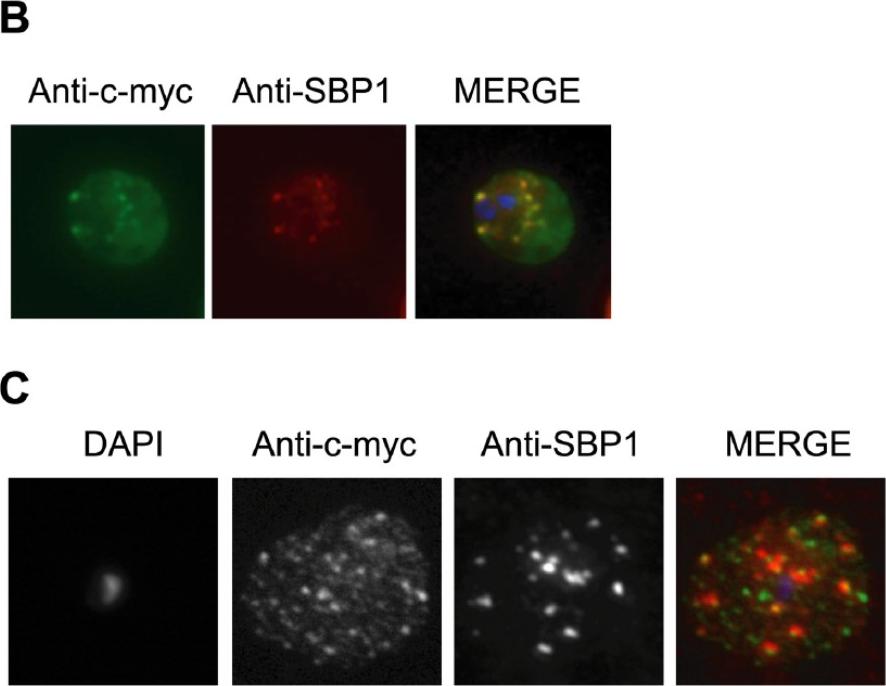
Immunolocalization of c-myc epitope-tagged PFE1605w PHIST protein within infected erythrocytes. B) Co-localization was assayed using rabbit polyclonal SBP1 followed by goat anti-rabbit IgG conjugated with Alexa 594 (red). C) The pattern of expression of c-myc epitope tagged PFE1605w PHIST protein is dependent on the fixation protocol. When infected erythrocytes are fixed in solution with 1% paraformaldehyde, 0.075% glutaraldehyde, then PHIST protein shows a broader punctate expression than the Maurer’s cleft marker, SBP1. At the late ring stage, PHIST proteins were observed exported to the erythrocyte cytoplasm, at least in partial co-localization with the marker of Maurer’s clefts, SBP1 (B). PFE1600w was abundantly observed in the ER of late stage parasites (data not shown); and it is possible that this localization represents aberrant trafficking due to over-expression of the transcript and thereby protein, perhaps due to the use of a heterologous promoter. The localization to the erythrocyte cytoplasm validates trafficking that is predicted by the presence of a PEXEL/HT motif.Moreira CK, Naissant B, Coppi A, Bennett BL, Aime E, Franke-Fayard B, Janse CJ, Coppens I, Sinnis P, Templeton TJ. The Plasmodium PHIST and RESA-Like Protein Families of Human and Rodent Malaria Parasites. PLoS One. 2016 11(3):e0152510
See original on MMP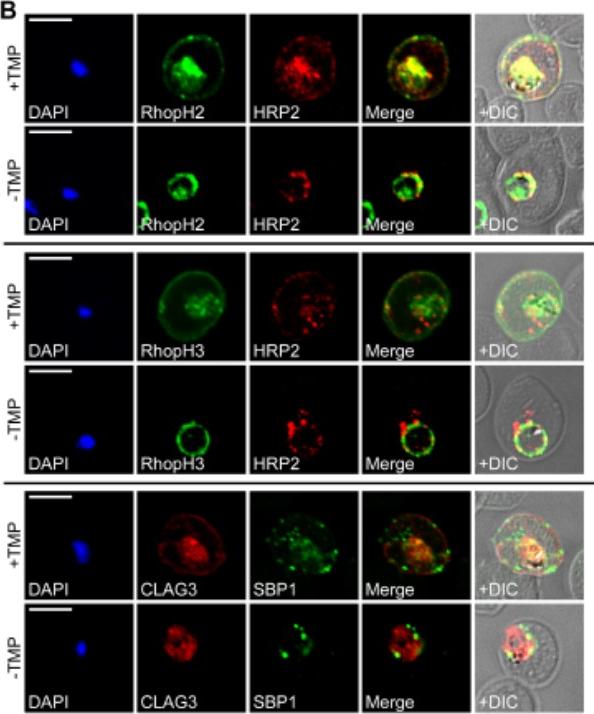
RhopH proteins are deposited in the PV and exported via PTEX. IFA of 13F10 showing export of RhopH proteins after growth with or without TMP for 48 h. Colocalization with exported HRP2 or SBP1, as indicated. Scale bars, 5 μm. Export of RhopH2 and RhopH3 were both fully blocked in trophozoite-stage parasites grown for 48 h without TMP. Colocalization with HRP2, a parasite protein carrying the canonical PEXEL export signal showed both proteins trapped in the vacuole upon PTEX knockdown. CLAG3 export requires PTEX activity as TMP removal prevented its movement out of the PV (bottom row); colocalization with SBP1, another PTEX substrate.Ito D, Schureck MA, Desai SA. An essential dual-function complex mediates erythrocyte invasion and channel-mediated nutrient uptake in malaria parasites. Elife. 2017 Feb 21;6.
See original on MMP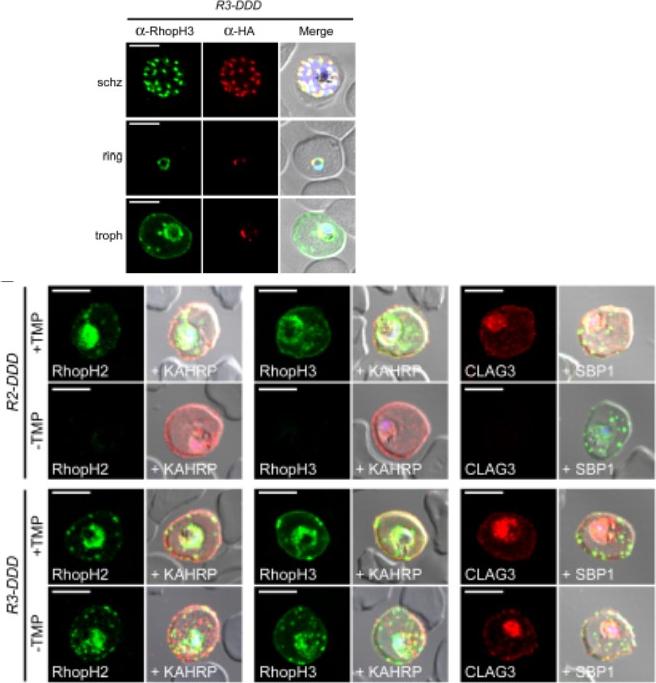
(D) IFA of R3-DDD (conditional RopH3 mutant) probed with indicated antibodies at each parasite stage. Note that both antibodies detect protein in schizont rhoptries, but that only the anti-RhopH antibody detects export to the host membrane in trophozoites. Scale bars, 5 μm. (E) Trophozoite-stage IFAs of indicated parasites cultivated with and without TMP for 48 h, showing that knockdown abolishes each member of the complex in R2-DDD (conditional RopH2 mutant) but not R3-DDD. Co-localization is shown with exported parasite proteins KAHRP or SBP1 (red or green in merge panels, respectively). Scale bars, 5 μm. The RhopH complex appears to be secreted into the PV (upper panel), suggesting that its member proteins must cross the bounding PVM to enter host cytosol and eventually reach the erythrocyte surface. While TMP removal rendered all three RhopH proteins undetectable in the R2-DDD parasite, these proteins and their transfer to trophozoites were preserved inR3-DDD.Ito D, Schureck MA, Desai SA. An essential dual-function complex mediates erythrocyte invasion and channel-mediated nutrient uptake in malaria parasites. Elife. 2017 Feb 21;6. PMID: 28221136.
See original on MMP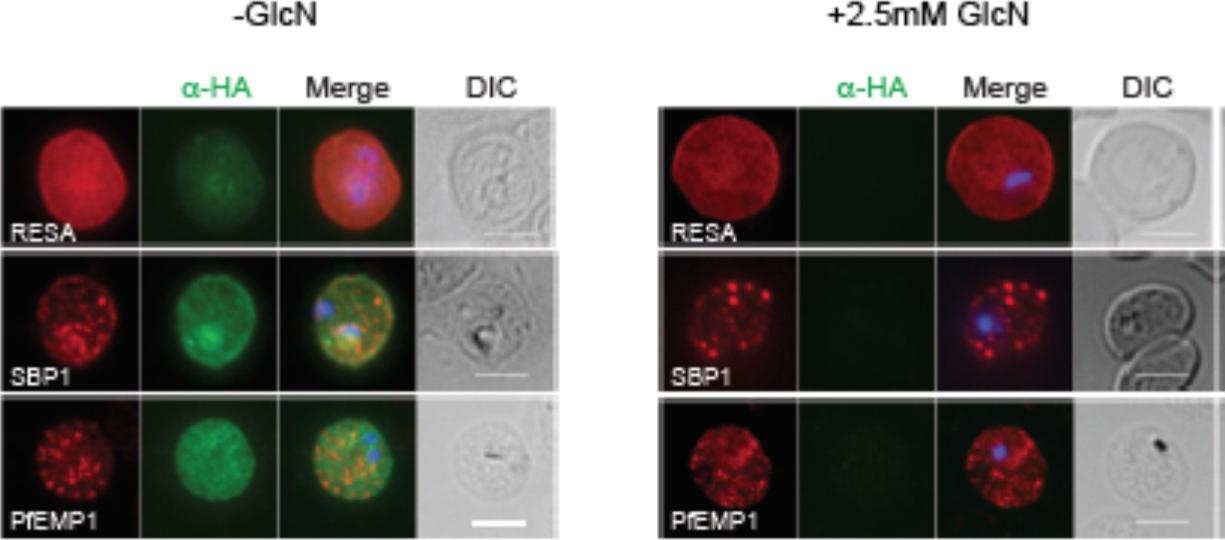
RhopH2 is not involved in the trafficking of exported proteins in the erythrocyte cytoplasm. (a) Representative IFAs of erythrocytes infected with RhopH2-HAglmS parasites grown in 0 mM or 2.5 mM GlcN using the indicated antibodies show trafficking of RESA, SBP1 and PfEMP1 is unaffected upon RhopH2 knockdown. Scale bar = 5 μm. No defect in the export of PfEMP1, or trafficking of either RESA to the erythrocyte membrane or SBP1 to the Maurer’s clefts was evident after knocking down RhopH2 expression with GlcN.Counihan N, Chisholm SA, Bullen HE, Srivastava A, Sanders PR, Jonsdottir TK, Weiss GE, Ghosh S, Crabb BS, Creek DJ, Gilson PR, de Koning-Ward TF. Plasmodium falciparum parasites deploy RhopH2 into the host erythrocyte to obtain nutrients, grow and replicate. Elife. 2017 Mar 2;6. pii: e23217.
See original on MMP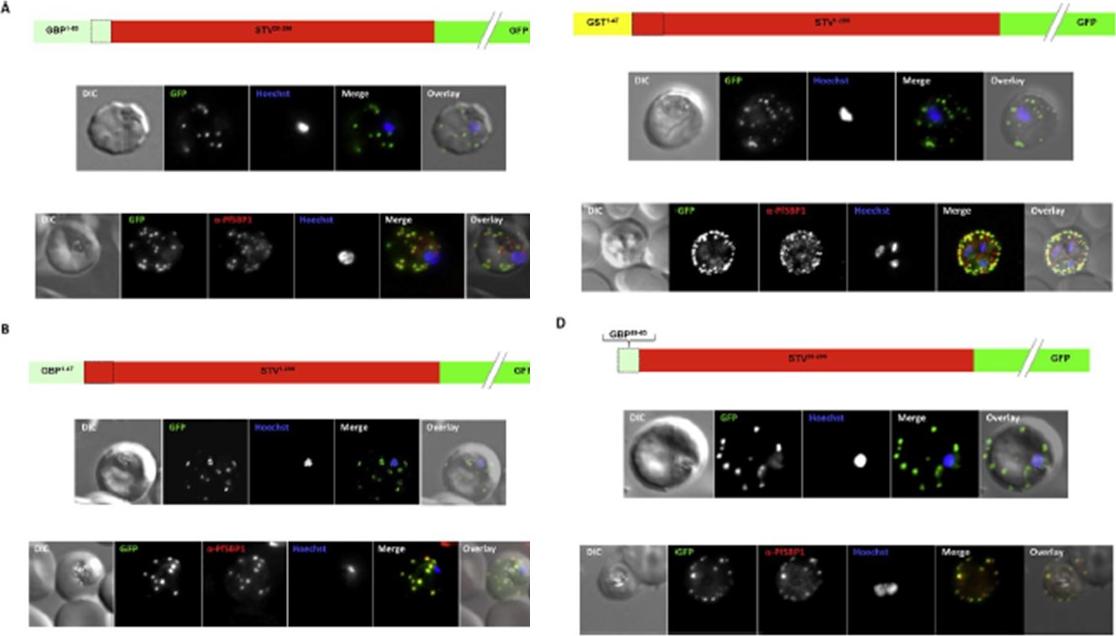
Effect of signal peptide type on export of a membrane protein. A schematic of eachconstruct is shown above the respective images. Upper panels show live cells, lowerpanels fixed cells. DIC, differential interference contrast; GFP, green fluorescent protein; Hoechst, nuclear staining. In merge and overlay: green, GFP; blue, Hoechst; red, anti-SBP1. Having excluded an important role for N-terminal extensions in the export of soluble proteins, we wished to study whether the C- domain from GBP130 is alone capable of driving entry of a membrane protein to the secretory pathway. For this reason we expressed a construct composed of the C- domain of GBP130 (aa48-65) fused to STEVOR lacking the endogenous signal peptide. Once again we observed a high level of export to the Maurer’s clefts, and co-localisation with PfSBP1Meyer C, Barniol L, Hiss JA, Przyborski JM. The N-terminal extension of the P. falciparum GBP130 signal peptide is irrelevant for signal sequence function. Int J Med Microbiol. 2017 S1438-4221(17)30195-9.
See original on MMPMore information
| PlasmoDB | PF3D7_0501300 |
| GeneDB | PF3D7_0501300 |
| Malaria Metabolic Pathways | Localisation images Pathways mapped to |
| Previous ID(s) | MAL5P1.14, PFE0065w |
| Orthologs | |
| Google Scholar | Search for all mentions of this gene |