PBANKA_0711900 heat shock protein 70 (HSP70)
Disruptability [+]
| Species | Disruptability | Reference | Submitter | |
|---|---|---|---|---|
| P. berghei ANKA |
Refractory |
PlasmoGEM (Barseq) | PlasmoGEM | |
| P. falciparum 3D7 |
Refractory |
USF piggyBac screen (Insert. mut.) | USF PiggyBac Screen | |
Mutant phenotypes [+]
None reported yet. Please press the '+' button above to add one.Imaging data (from Malaria Metabolic Pathways)
Immunoelectron micrographs of extracellular merozoites of P. falciparum. Merozoites were incubated with (A) mouse antiserum to bacterial lysates of clone C7, (B) mouse antiserum raised to E. coli containing the cloning vector pUC8 (negative control). Hsp70 is localized at the surface of the merozoite. The location of p75 on the merozoite surface is unusual for an hsp-related protein, as most of these are normally intracellular.Reprinted by permission from Macmillan Publishers Ltd: Ardeshir F, Flint JE, Richman SJ, Reese RT. A 75 kd merozoite surface protein of Plasmodium falciparum which is related to the 70 kd heat-shock proteins. EMBO J. 1987 6:493-9. Copyright 2009. Copyright Elsevier 2011.
See original on MMP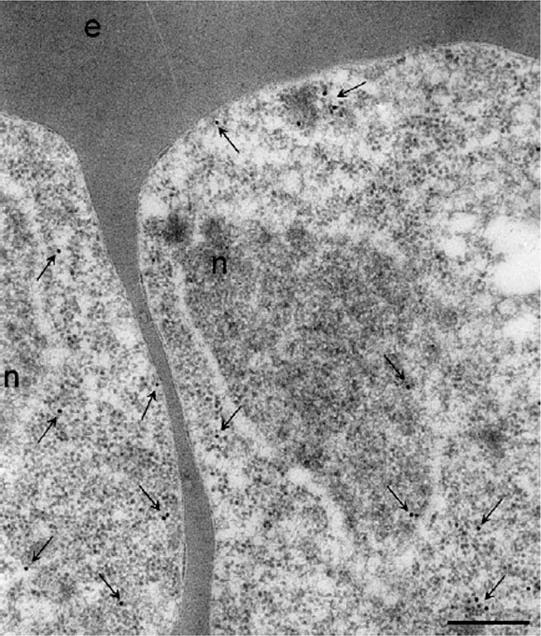
Localisation of PfHsp70-1 in trophozoites-infected erythrocytes by immunoEM. PfHsp70-1 localisation was visualised by gold-conjugated anti-rabbit secondary antibodies. PfHsp70-1 was detected in the nucleus and cytoplasm of the parasites. PfHsp70-1 was often detected at the periphery of the parasite cytoplasm. The nucleus (n) and the erythrocyte (e) are indicated. Arrows indicate some of the immunostaining. Size bar, 200 nm.Pesce ER, Acharya P, Tatu U, Nicoll WS, Shonhai A, Hoppe HC, Blatch GL. The Plasmodium falciparum heat shock protein 40, Pfj4, associates with heat shock protein 70 and shows similar heat induction and localisation patterns. Int J Biochem Cell Biol. 2008 40:2914-26.
See original on MMP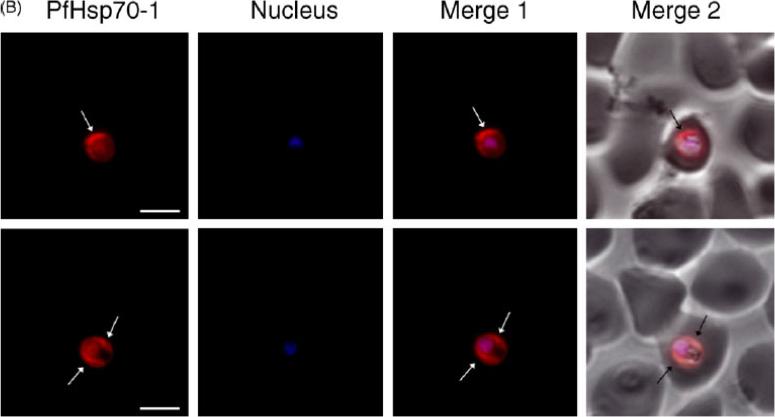
Expression and localisation of PfHsp70-1 in trophozoite-infected erythrocytes. The distribution of PfHsp70-1 was visualised using anti-rabbit IgG-TRITC secondary antibodies and nuclei were visualised by DAPI staining. PfHsp70-1 localises mainly at the periphery of the parasite cytoplasm and in the nucleus. PfHsp70-1, images were captured under the TRITC filter and the fluorescence is pseudocoloured red. Nucleus, images were captured under UV light and the fluorescence is pseudocoloured blue. Merge 1, combination of the TRITC and DAPI images. Merge 2, combination of merge 1 and the corresponding phase contrast image. Arrows indicate localisation of PfHsp70-1 at the periphery of the parasite cytoplasm. Size bars, 5mm.Pesce ER, Acharya P, Tatu U, Nicoll WS, Shonhai A, Hoppe HC, Blatch GL. The Plasmodium falciparum heat shock protein 40, Pfj4, associates with heat shock protein 70 and shows similar heat induction and localisation patterns. Int J Biochem Cell Biol. 2008 40:2914-26. Copyright Elsevier 2011.
See original on MMP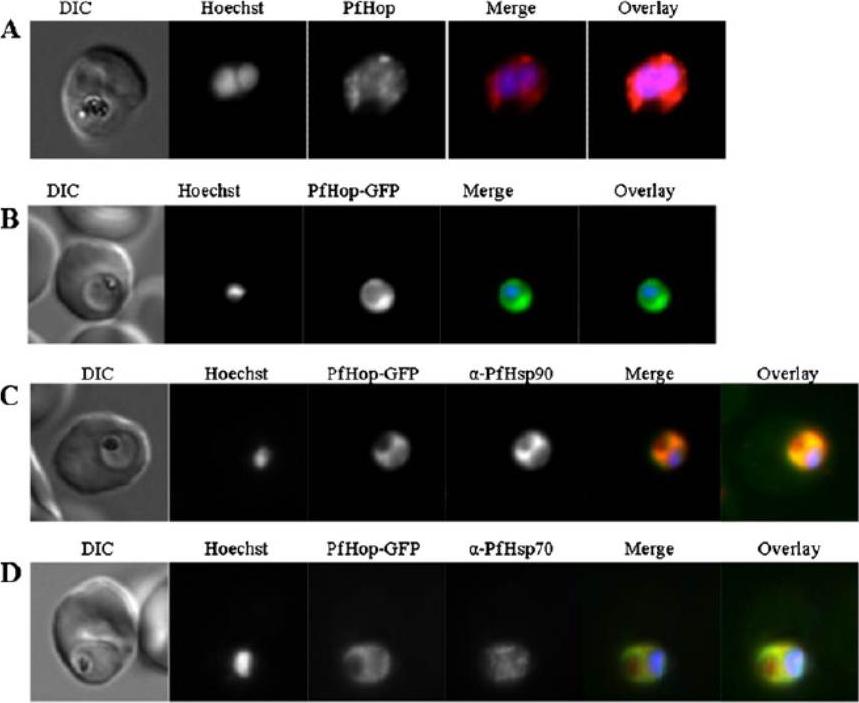
Localisation and expression of PfHop-GFP by parasites in human red blood cells. a Localisation of PfHop in P. falciparum cells; panels show a DIC image, nuclear stain (Hoechst), distribution of PfHop, merge and overlay. b Panels show a DIC image, nuclear stain (Hoechst), distribution of PfHop-GFP, merge and overlay. c Panels show a DIC image, nucleus (Hoechst), distribution of PfHop-GFP, distribution of PfHsp90, merge and overlay for PfHsp90–PfHop-GFP co-localisation. d Panels show DIC image, stain (Hoechst), distribution of PfHop-GFP, distribution of PfHsp70, merge and overlay. PfHop displayed a similar cytosolic localisation profile to PfHsp90 (b), suggesting that the two proteins may associate. Although, PfHop-GFP and PfHsp70 exhibited overlapping cytosolic co-localisation signals, the PfHsp90–PfHop-GFP co-localisation signal was more uniform than that for PfHsp70–PfHop-GFP (b, c). It is possible that PfHop associates more closely with PfHsp90 than with PfHsp70. Gitau GW, Mandal P, Blatch GL, Przyborski J, Shonhai A. Characterisation of the Plasmodium falciparum Hsp70-Hsp90 organising protein (PfHop). Cell Stress Chaperones. 2011 17(2):191-202.
See original on MMP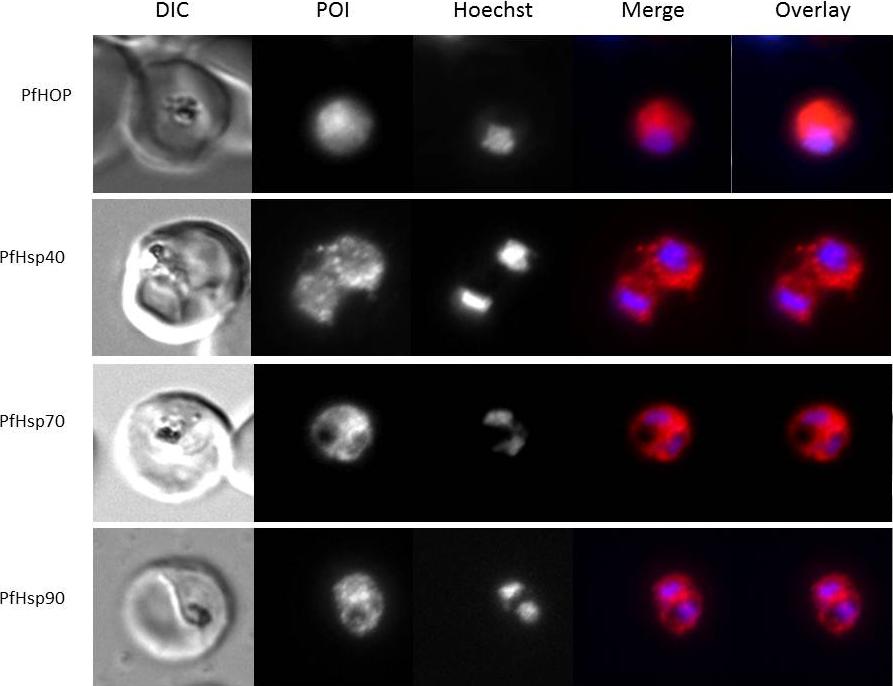
Immunofluorescence staining to detect Hsp40, HSP70, HSP90 and HOP was conducted on trophozoite stage P. falciparum-infected erythrocytes. Panels show from left to right a DIC image, distribution of protein of interest (POI), nuclear stain (Hoechst), merge and overlay (localization relative to the parasite nucleus and phase-contrast image). All proteins showed cytosolic localization.Botha M, Chiang AN, Needham PG, Stephens LL, Hoppe HC, Külzer S, Przyborski JM, Lingelbach K, Wipf P, Brodsky JL, Shonhai A, Blatch GL. Plasmodium falciparum encodes a single cytosolic type I Hsp40 that functionally interacts with Hsp70 and is upregulated by heat shock. Cell Stress Chaperones. 2011 16(4):389-401. Pictures were kindly provided by Jude Przyborski.
See original on MMP
Co-localization assays of PfSUMO antiserum with nuclear marker PfSir2, cytoplasmic marker, PfHsp70-1 and Maurer’s clefts marker, Pf332 reveal compartmentalization. Size bar corresponds to 1 mm.Issar N, Roux E, Mattei D, Scherf A. Identification of a novel post-translational modification in Plasmodium falciparum: protein sumoylation in different cellular compartments. Cell Microbiol. 2008 10:1999-2011.
See original on MMP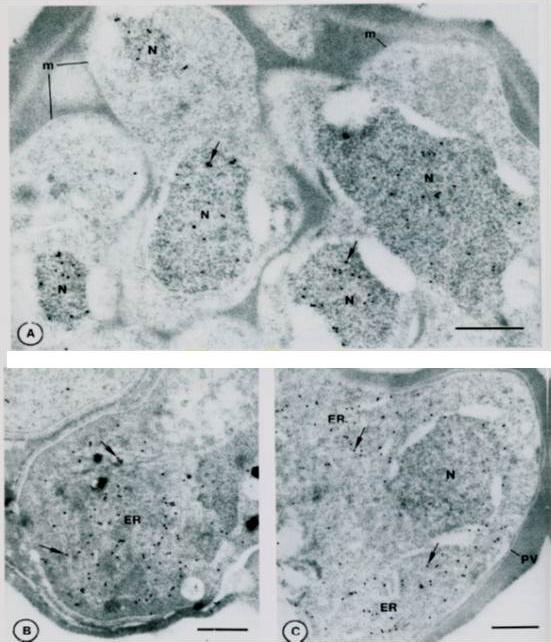
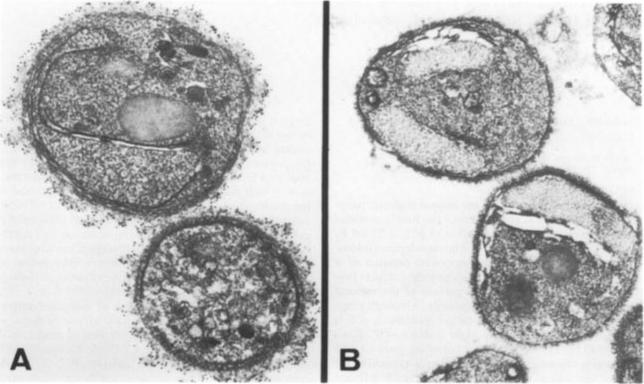
Immuno-gold localization of Pfhsp and Pfgrp. A. Sections of a mature segmenter with rappit antiserum against Pfhsp fusion protein and protein A-gold. The nuclei (N) of budding merozoites (m) are labeled diffusely with colloidal gold particles (arrows). B. Section of mature gametocyte incubated witrh antiserum against Pfgrp peptide and protein A-gold.. Label (arrows) is associated with membranes of the ER. C. Sections of a schizont. Lable (arrows) is associated with discrete areas of the schizont cytoplasm that correspond to ER. Not absence of label on the nucleus (N) and parasitophorous vacuole (PV). Bar – 0.5 mm.Kumar N, Koski G, Harada M, Aikawa M, Zheng H. Induction and localization of Plasmodium falciparum stress proteins related to the heat shock protein 70 family. Mol Biochem Parasitol. 1991 48:47-58. Copyright Elsevier 2009.
See original on MMP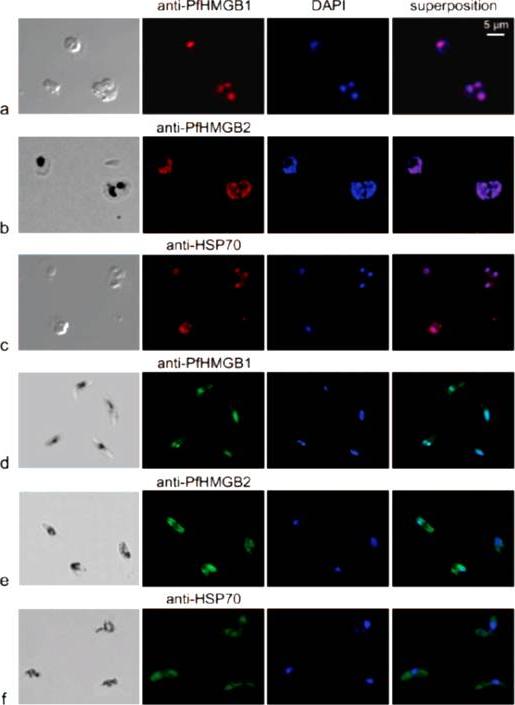
Immunofluorescence localization of PfHMGB1 and PfHMGB2 in asexual (a, b, and c) and sexual (d, e, and f) stages of Plasmodium erythrocytic development. Paraformaldehyde-fixed parasites were labeled with mouse anti-PfHMGB1 and anti-PfHMGB2 antibodies (1:200) and FITC-conjugated anti-mouse IgG (1:100); DNA was stained with DAPI (1:100). Merged fluorescent signals are shown in the “superposition” column. Cells were visualized by phase-contrast (a and c) or transmission (b, d, e, and f) microscopy. Panels: a, trophozoites; b, trophozoite and schizont; c, trophozoites; d to f, gametocytes. Anti-PfHMGB and anti-HSP70 fluorescence is red for panels a to c and green for panels d to f. We also compared the localizations of both factors in asexual (red immunofluorescence) and gametocyte (green immunofluorescence) stages. As already mentioned, the two PfHMGB factors (lanes a and b) appeared to be located mainly in the nucleus of the asexual stages (rings, trophozoites, and schizonts), whereas the HSP70 protein (lane c) was also found in the parasite cytoplasm. Surprisingly, in addition to its nuclear localization, PfHMGB2 could also be readily detected within the cytoplasm of different stages (IV and V) of gametocytes (lanes e), as also observed for the HSP70 protein (lane f), whereas PfHMGB1 was associated mainly with the nucleus of gametocytes, as in asexual parasites (lanes d and a).Briquet S, Boschet C, Gissot M, Tissandié E, Sevilla E, Franetich JF, Thiery I, Hamid Z, Bourgouin C, Vaquero C. High-mobility-group box nuclear factors of Plasmodium falciparum. Eukaryot Cell. 2006 5(4):672-82.
See original on MMP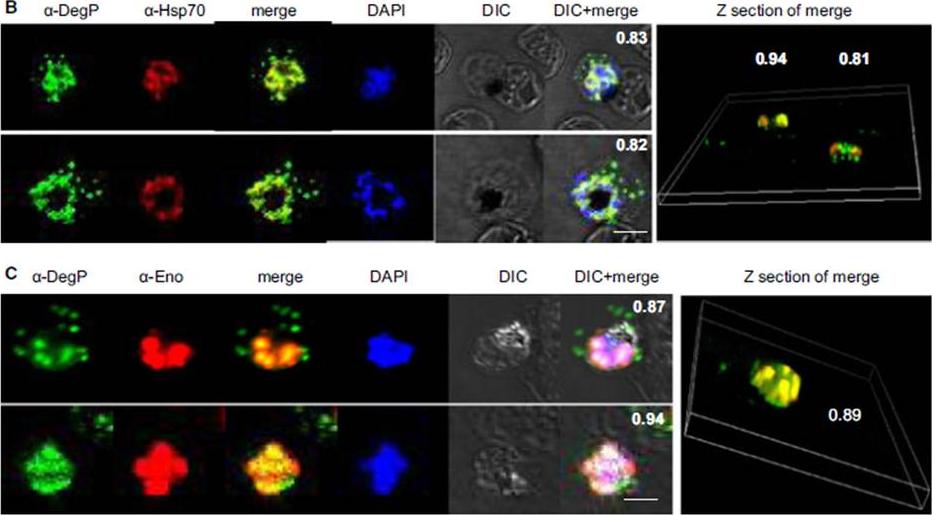
PfDegP exists in a multimeric complex, and is associated with parasite proteins Hsp70 and Eno. Parasites were stained with anti-DegP and anti-Hsp70 (B) or anti-Eno (C) antiserum; parasite nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI). Fluorescence signals from DegP and Hsp70 or Eno antibodies showed significant merging, with a co-localization coefficient > 0.80.Sharma S, Jadli M, Singh A, Arora K, Malhotra P. A secretory multifunctional serine protease, DegP of Plasmodium falciparum, plays an important role in thermo-oxidative stress, parasite growth and development. FEBS J. 2014 Mar;281(6):1679-99.
See original on MMP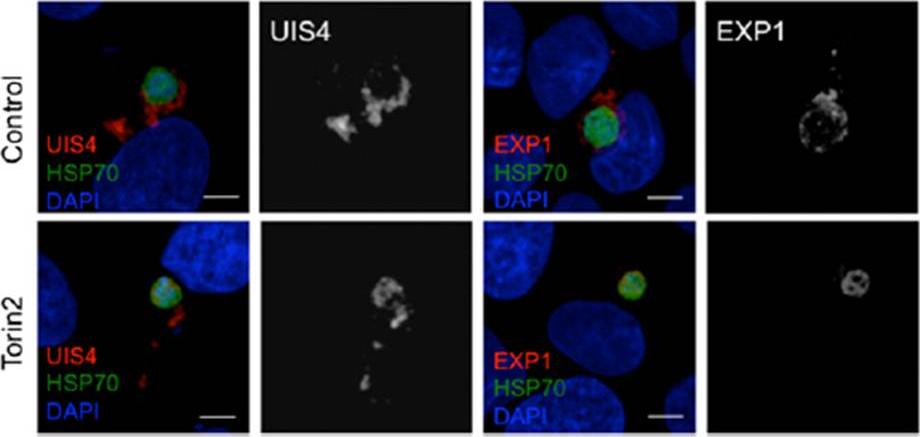
Effect of Torin2 treatment on the PVM and UIS4 localization in young liver stage trophozoites. (C) Effect of Torin2 treatment on UIS4 and EXP1 during early liver stage schizogony. UIS4 and EXP1 were localized almost completely outside of the parasite soma in control. Torin2 treatment led to a dramatic mislocalization of UIS4 and EXP1, with signal largely found in the parasite soma.Hanson KK, Ressurreição AS, Buchholz K, Prudêncio M, Herman-Ornelas JD, Rebelo M, Beatty WL, Wirth DF, Hänscheid T, Moreira R, Marti M, Mota MM. Torins are potent antimalarials that block replenishment of Plasmodium liver stage parasitophorous vacuole membrane proteins. Proc Natl Acad Sci U S A. 2013 110(30):E2838-47.
See original on MMP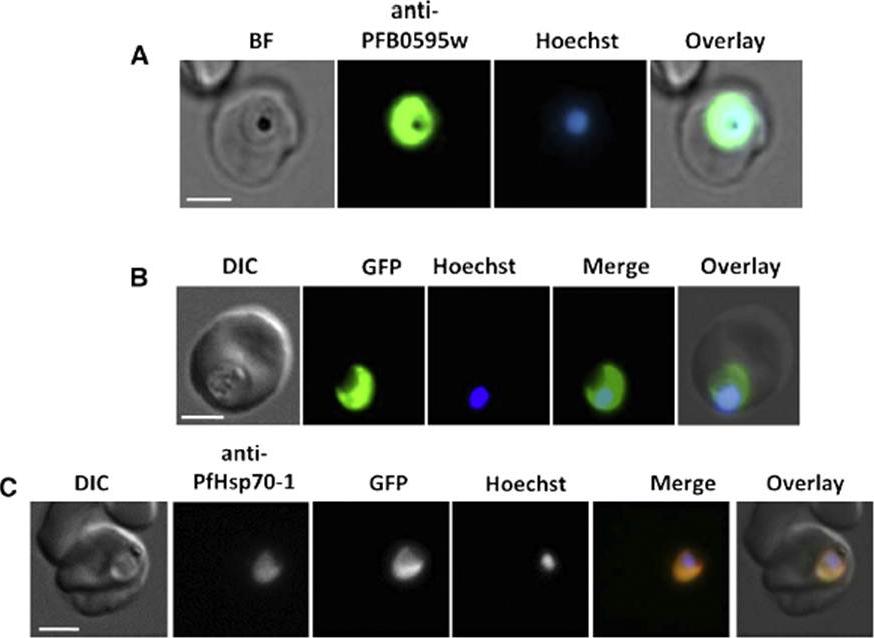
PFB0595w is localized to the parasite cytosol. (A) Immunolocalization of PFB0595w using the anti-PFB0595w antibody on fixed P. falciparum 3D7-infected erythrocytes stained with Hoechst 33258 (to visualize the nucleus) BF, bright field. (B) Localization of the GFP signal through live cell imaging of PFB0595w-GFP transfectants stained with Hoechst 33528. DIC, differential interference contrast; Merge, PFB0595w-GFP plus Hoechst 33258 channel; Overlay, DIC plus merge. (C) Indirect immunofluorescence localization of GFP and PfHsp70-1 in PFB0595w-GFP transfectants using the anti-PfHsp70-1 antibody. Merge, anti-PfHsp70-1 plus PFB0595w-GFP and Hoechst 33258;Overlay, DIC plus merge.Njunge JM, Mandal P, Przyborski JM, Boshoff A, Pesce ER, Blatch GL. PFB0595w is a Plasmodium falciparum J protein that co-localizes with PfHsp70-1 and can stimulate its in vitro ATP hydrolysis activity. Int J Biochem Cell Biol. 2015 62:47-53.
See original on MMP
Sub-cellular distribution and localization of PfHsp60 (continued). (A) IFA of localization PfHsp60 in P. falciparum infected erythrocytes with (i) Propidium iodide (Nuclear marker), (ii) Mitotracker, (iii) PfCytochrome C (Mitochondrial marker), (iv) PfUROD (Apicoplast marker), (v) PfPlasmepsin V (PfPMV, endoplasmic reticulum marker), and (vi) PfHsp70-1 (Cytosol marker). PfHsp60 is stained with FITC-conjugated secondary antibody in all parts, while PfUROD, PfPlasmepsin V, PfCytochrome C, and PfHsp70-1 are stained with TRITC-cojugated secondary antibody in (iii), (iv), and (v). In all images, white arrow indicates the erythrocyte membrane. Part (i) shows that PfHsp60 is present only in the extra-nuclear compartment. Parts (ii) and (iii) show that PfHsp60 is present in the cytoplasm as well as in the mitochondria. The yellow signal in the merged panel shows that there is some co-localization. Part (iv) shows a lack of signal overlap with PfUROD in the merge panel. Part (v) shows a lack of signal overlap with PfPlasmepsin V in the merge panel, which rules out its localization in endoplasmic reticulum. Part (vi) shows that there is co-localization between the cytosolic protein PfHsp70-1 and PfHsp60, which indicates that PfHsp60 indeed accumulates in the cytosolPadma Priya P, Grover M, Tatu US, Natarajan V. Characterization of Precursor PfHsp60 in Plasmodium falciparum Cytosol during Its Asexual Development in Human Erythrocytes. PLoS One. 2015 Aug 28;10(8):e0136401. PMID:
See original on MMPMore information
| PlasmoDB | PBANKA_0711900 |
| GeneDB | PBANKA_0711900 |
| Malaria Metabolic Pathways | Localisation images Pathways mapped to |
| Previous ID(s) | PB000817.02.0, PB300582.00.0, PBANKA_071190 |
| Orthologs | PCHAS_0721000 , PF3D7_0818900 , PKNH_1312700 , PVP01_0515400 , PVX_089425 , PY17X_0712100 |
| Google Scholar | Search for all mentions of this gene |